- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
AQA A Level Chemistry复习笔记5.5.2 pH & [H⁺]
pH & [H⁺]
- The acidity of an aqueous solution depends on the number of H+ (H3O+) ions in solution
- The pH is defined as:
pH = -log[H+]
-
- where [H+] is the concentration of hydrogen ions in mol dm–3
- Similarly, the concentration of H+ of a solution can be calculated if the pH is known by rearranging the above equation to:
[H+] = 10-pH
- The pH scale is a logarithmic scale with base 10
- This means that each value is 10 times the value below it. For example, pH 5 is 10 times more acidic than pH 6.
- pH values are usually given to 2 decimal places
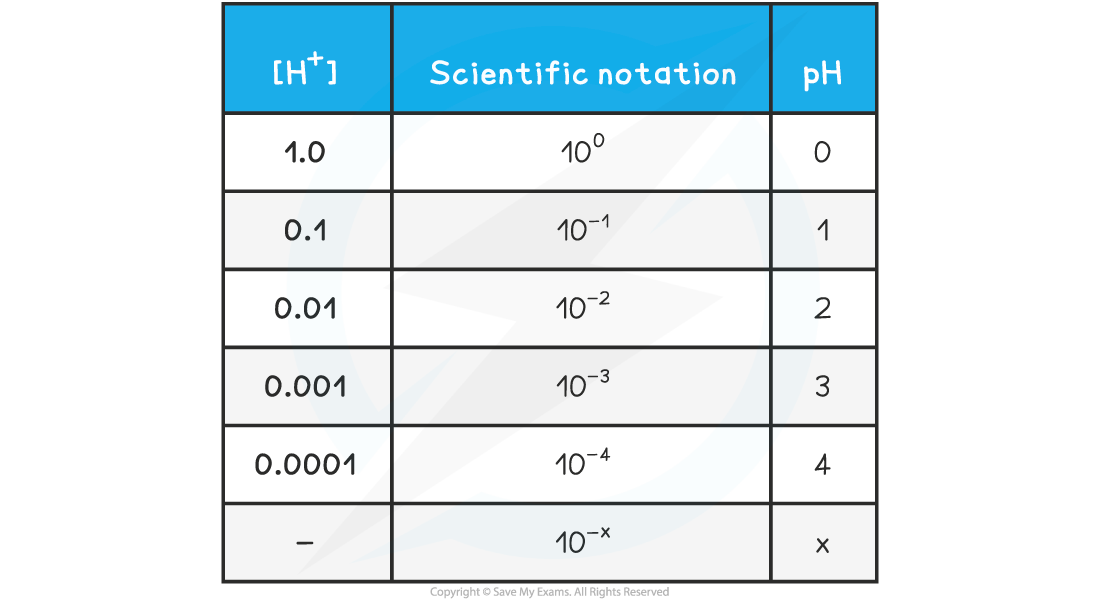
- The relationship between concentration is easily seen on the following table
pH & [H+] Table
Worked Example
pH and H+ calculations
Question 1: Find the pH when the hydrogen concentration is 1.60 x 10-4 mol dm-3
Question 2: Find the hydrogen concentration when the pH is 3.10
Answer
Answer 1:
The pH of the solution is:
pH = -log[H+]
= -log 1.6 x 10-4
= 3.80
Answer 2:
The hydrogen concentration can be calculated by rearranging the equation for pH
pH = -log[H+]
[H+] = 10-pH
= 10-3.10
= 7.94 x 10-4 mol dm-3
Worked Example
Powers of 1010.0 cm3 of an aqueous solution of an acid of pH = 1.0 is mixed with 990.0 cm3 of distilled water. What is the pH of the final solution?
A. 1
B. 2
C. 3
D. 10
Answer
The correct option is C.
-
- The total volume after dilution is 1000.0 cm3 so the concentration of H+ has been reduced by a factor of 100 or 10-2, which means an increase of 2 pH units
- The final solution is therefore pH 3
pH of Strong Acids
Strong acids
- Strong acids are completely ionised in solution
HA (aq) → H+ (aq) + A- (aq)
- Therefore, the concentration of hydrogen ions, H+, is equal to the concentration of acid, HA
- The number of hydrogen ions formed from the ionisation of water is very small relative to the [H+] due to ionisation of the strong acid and can therefore be neglected
- The total [H+] is therefore the same as the [HA]
Worked Example
What is the pH of 0.01 mol dm-3 hydrochloric acid?
Answer
[HCl] = [H+] = 0.01 mol dm-3
pH = - log[H+]
pH = - log[0.01] = 2.00
The pH of dibasic acids
- Dibasic or diprotic acids have two replaceable protons and will react in a 1:2 ratio with bases
- Sulfuric acid is an example
H2SO4 (aq) + 2NaOH (aq) → Na2SO4 (aq) + 2H2O (l)
- You might think that being a strong acid it is fully ionised so the concentration of the hydrogen is double the concentration of the acid
- This would mean that 0.1 mol dm-3 would be 0.2 mol dm-3 in [H+] and have a pH of 0.69
- However, measurements of the pH of 0.1 mol dm-3 sulfuric acid show that it is actually about pH 0.98, which indicates it is not fully ionised
- The ionisation of sulfuric acid occurs in two steps
H2SO4 → HSO4- + H+
HSO4- ⇌ SO42- + H+
- Although the first step is thought to be fully ionised, the second step is suppressed by the abundance of hydrogen ions from the first step creating an equilibrium
- The result is that the hydrogen ion concentration is less than double the acid concentration
Exam Tip
Make sure you know how to use the antilog (base 10) feature on your calculator. On most calculators it is the 10x button, but on other models it could be LOG-1, ALOG or even a two-button sequence such as INV + LOG
转载自savemyexams

最新发布
© 2026. All Rights Reserved. 沪ICP备2023009024号-1









