- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
AQA A Level Chemistry复习笔记5.4.4 Commercial Cells
Commercial Cells
- Electrochemical cells can be used as a commercial source of electrical energy
- Cells can be non-rechargeable (irreversible), rechargeable or fuel cells
- Type of cells used in commercial applications depend on
- the voltage required
- the current needed
- the size of the cell
- the cost
- Although it is commonly used incorrectly, the term battery should be used to refer to a collection of cells
- A car battery is correct, because it is a collection of six cells joined together
Non-rechargeable cells
The Daniell cell
- The Daniell cell was one of the earliest electrochemical cells and consisted of a simple metal-metal ion system
- It was invented by British chemist John Daniell in 1836
- The cell consists of
- a zinc rod immersed in a solution of zinc sulfate
- a copper cylinder filled with copper sulfate solution
- a porous pot that separates the copper sulfate from the zinc sulfate
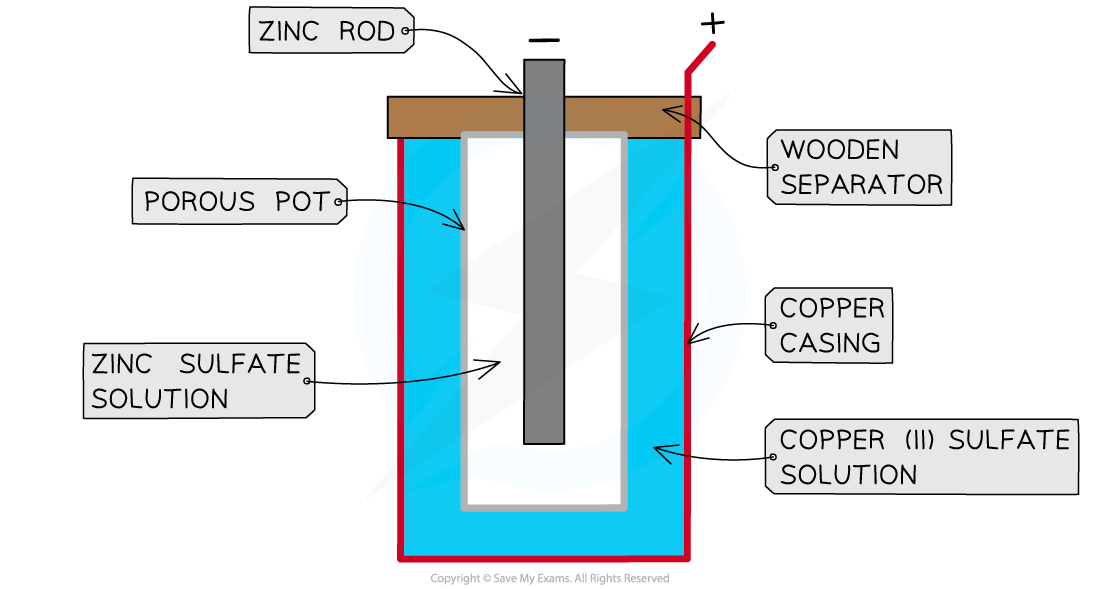
A Daniell cell
- The zinc acts as the negative electrode and the copper is the positive electrode
- The half-cell reactions are
Zn (s) → Zn2+ (aq) + 2e- Eꝋ = -0.76 V
Cu2+ (aq) + 2e- → Cu (s) Eꝋ = +0.34 V
- The cell generates an emf of 1.1 V and the overall reaction is
Zn (s) + CuSO4 (aq)→ Cu (s) + ZnSO4 (aq) Eꝋcell = +1.10 V
- However, the cell is impractical to use as a portable device because of the hazardous liquids in the cell
Zinc-carbon cells
- Zinc-carbon cells are the most common type of non-rechargeable cells, consisting of
- a zinc casing which acts as the negative electrode
- a paste of ammonium chloride which acts as an electrolyte as well as the positive electrode
- a carbon rod which acts as an electron carrier in the cell
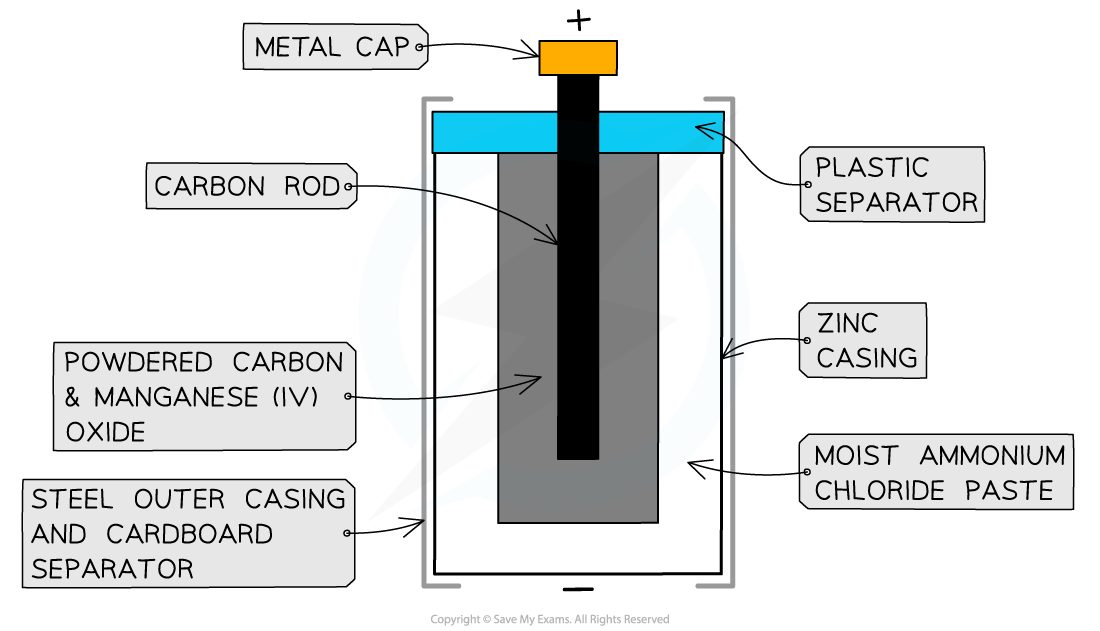
The zinc-carbon cell
- The half-cell reactions are
Zn (s) → Zn2+ (aq) + 2e- Eꝋ = -0.76 V
2NH4+ (aq) + 2e- → 2NH3 (g) + H2 (g) Eꝋ = +0.74 V
- The cell generates an emf of 1.50 V and the overall reaction is
2NH4+ (aq) + Zn (s) → 2NH3 (g) + H2 (g) + Zn2+ (aq) Eꝋcell = +1.50 V
- As the cell discharges, the zinc casing eventually wears away and the corrosive contents of the electrolyte paste can leak out, which is an obvious disadvantage of zinc-carbon cells
- The cell provides a small current and is relative cheap compared to other cells
- Extra long life cells have a similar chemistry, but supply a higher current and use zinc chloride in the paste; they are suitable for torches, radios and clocks
- Another variation on the cell uses an alkaline paste in the electrolyte and they have a much longer operating life, but are noticeably more expensive than regular zinc-carbon cells
Rechargeable Cells
- Rechargeable cells employ chemical reactions which can be reversed by applying a voltage greater than the cell voltage, causing electrons to push in the opposite direction
- There are many types of rechargeable cells, but common ones include lead-acid batteries, NiCad cells and lithium cells which are covered in more detail in the next section
Lead-acid batteries
- Lead-acid batteries consist of six cells joined together in series
- The cells use lead metal as the negative electrode and and lead(IV) oxide as the positive electrode
- The electrolyte is sulfuric acid
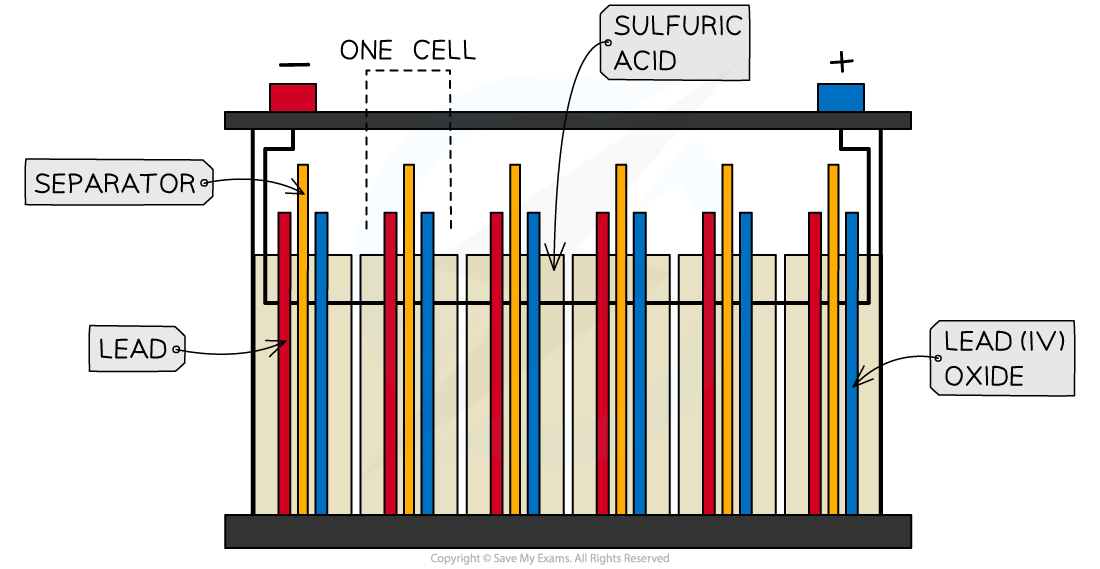
A lead-acid battery
- The half-cell reactions are
Pb (s) + SO42- (aq) → PbSO4 (s) + 2e- Eꝋ = -0.36 V
PbO2 (s) + 4H+ (aq) + SO42- (aq) + 2e- → PbSO4 (s) + 2H2O (l) Eꝋ = +1.70 V
- The cell generates an emf of about 2 V and the overall reaction is
PbO2 (s) + 4H+ (aq) + 2SO42- (aq) + Pb (s) → 2PbSO4 (s) + 2H2O (l) Eꝋcell = +2.06 V
- In a commercial car battery, the six cells in series give a combined voltage of about 12 V
- When the car is in motion, the generator provides a push of electrons that reverses the reaction and regenerates lead and lead(IV) oxide
- Lead-acid batteries are designed to produce a high current for a short period of time, hence their use in powering a starter motor in car engines
- The disadvantage of lead-acid batteries is that:
- They are very heavy
- They contain toxic materials: lead and lead(IV) oxide
- The sulfuric acid electrolyte is very corrosive
- This presents challenges of disposal when lead-acid batteries come to the end of their useful life
NiCad cells
- NiCad stands for nickel-cadmium and these cells are available in many standard sizes and voltages so they can replace almost any application of traditional zinc-carbon cells
- Although they are more expensive cells, the fact they can be recharged hundreds of times means they are commercially viable
- The positive electrode consists of cadmium and the negative electrode is made of a nickel(II) hydroxide-oxide system
- The half-cell reactions are
Cd (s) + 2OH- (aq) → Cd(OH)2 (s) + 2e-Eꝋ = -0.82 V
NiO(OH) (s) + H2O (l) + e- → Ni(OH)2 (s) + OH- (aq) Eꝋ = +0.38 V
- The overall reaction in the cell is
2NiO(OH) (s) + 2H2O (l) + Cd (s) → 2Ni(OH)2 (s) + Cd(OH)2 (s) Eꝋ = +1.2 V
- Cadmium is a toxic metal so the disposal of old NiCad cells is also an environmental issue
转载自savemyexams

最新发布
© 2025. All Rights Reserved. 沪ICP备2023009024号-1









