- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
AQA A Level Chemistry复习笔记5.4.3 Predicting Reactions
Predicting Reactions
Direction of electron flow
- The direction of electron flow can be determined by comparing the Eꝋ values of two half-cells in an electrochemical cell
Cl2 (g) + 2e- ⇌ 2Cl- (aq) Eꝋ = +1.36 V
Cu2+ (aq) + 2e- ⇌ Cu (s) Eꝋ = +0.34 V
- The Cl2 more readily accept electrons from the Cu2+/Cu half-cell
- This is the positive pole
- Cl2 gets more readily reduced
- The Cu2+ more readily loses electrons to the Cl2/Cl- half-cell
- This is the negative pole
- Cu2+ gets more readily oxidised
- The electrons flow from the Cu2+/Cu half-cell to the Cl2/Cl- half-cell
- The flow of electrons is from the negative pole to the positive pole
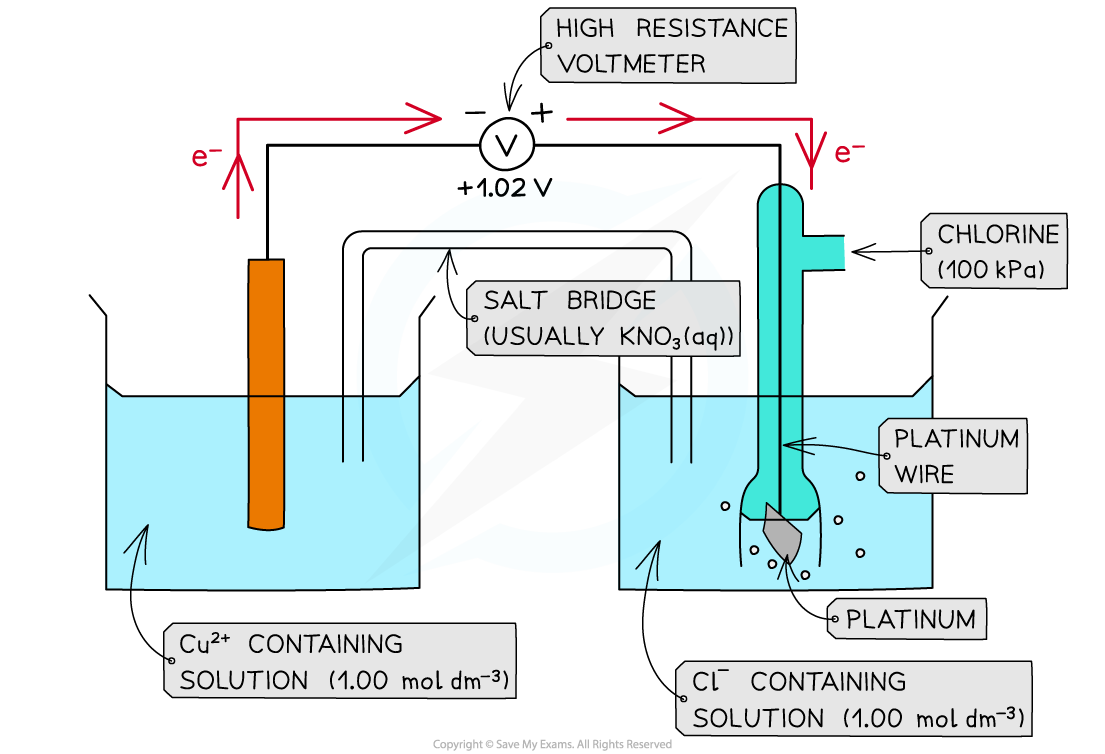
The electrons flow through the wires from the negative pole to the positive pole
Feasibility
- The Eꝋ values of a species indicate how easily they can get oxidised or reduced
- The more positive the value, the easier it is to reduce the species on the left of the half-equation
- The reaction will tend to proceed in the forward direction
- The less positive the value, the easier it is to oxidise the species on the right of the half-equation
- The reaction will tend to proceed in the backward direction
- A reaction is feasible (likely to occur) when the Ecellꝋ is positive
- For example, two half-cells in the following electrochemical cell are:
Cl2 (g) + 2e- ⇌ 2Cl- (aq) Eꝋ = +1.36 V
Cu2+ (aq) + 2e- ⇌ Cu (s) Eꝋ = +0.34 V
- Cl2 molecules are reduced as they have a more positive Eꝋ value
- The chemical reaction that occurs in this half cell is:
Cl2 (g) + 2e- → 2Cl- (aq)
- Cu2+ ions are oxidised as they have a less positive Eꝋ value
- The chemical reaction that occurs in this half cell is:
Cu (s) → Cu2+ (aq) + 2e-
- The overall equation of the electrochemical cell is (after cancelling out the electrons):
Cu (s) + Cl2 (g) → 2Cl- (aq) + Cu2+ (aq)
OR
Cu (s) + Cl2 (g) → CuCl2 (s)
- The forward reaction is feasible (spontaneous) as it has a positive Eꝋ value of +1.02 V ((+1.36) - (+0.34))
- The backward reaction is not feasible (not spontaneous) as it has a negative Eꝋ value of -1.02 ((+0.34) - (+1.36))

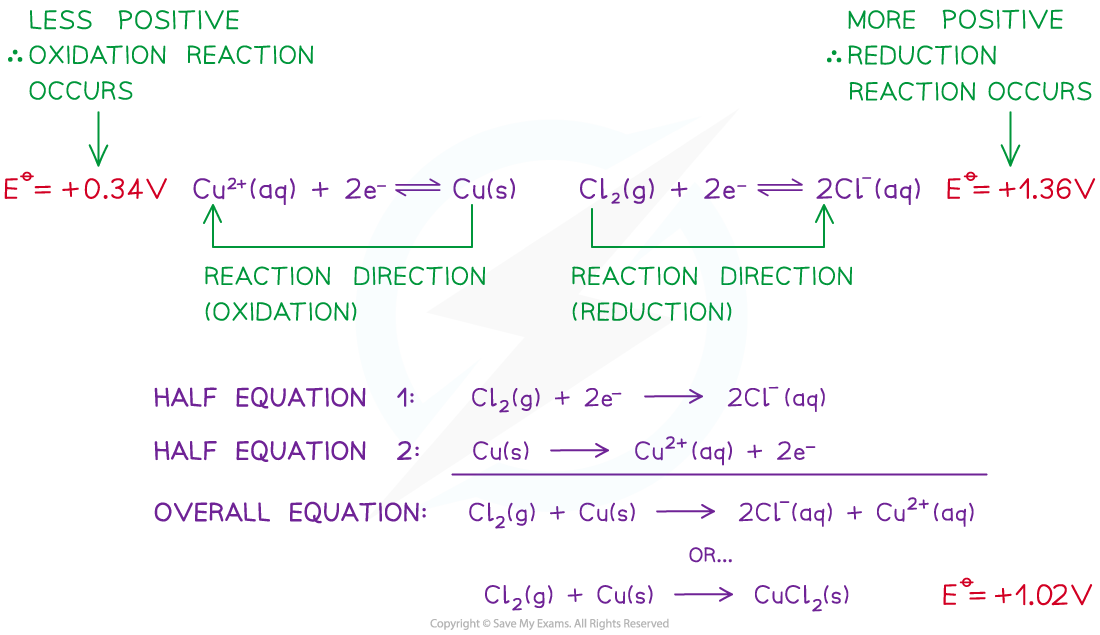
A reaction is feasible when the standard cell potential Eꝋ is positive
Exam Tip
Remember that the electrons only move through the wires in the external circuit and not through the electrolyte solution.
转载自savemyexams

早鸟钜惠!翰林2025暑期班课上线

最新发布
© 2025. All Rights Reserved. 沪ICP备2023009024号-1








