- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
AQA A Level Chemistry复习笔记5.3.3 Changes in Temperature & Pressure
Changes in Temperature & Pressure
- We can apply Le Chatelier's Principle to gaseous equilibria in the same way it is applied to aqueous systems
- Here's a reminder of how the principle works
Le Chatelier’s principle
- Le Chatelier’s principle says that if a change is made to a system in dynamic equilibrium, the position of the equilibrium moves to counteract this change
- The principle is used to predict changes to the position of equilibrium when there are changes in temperature, pressure or concentration
Predicting changes in Temperature & Pressure
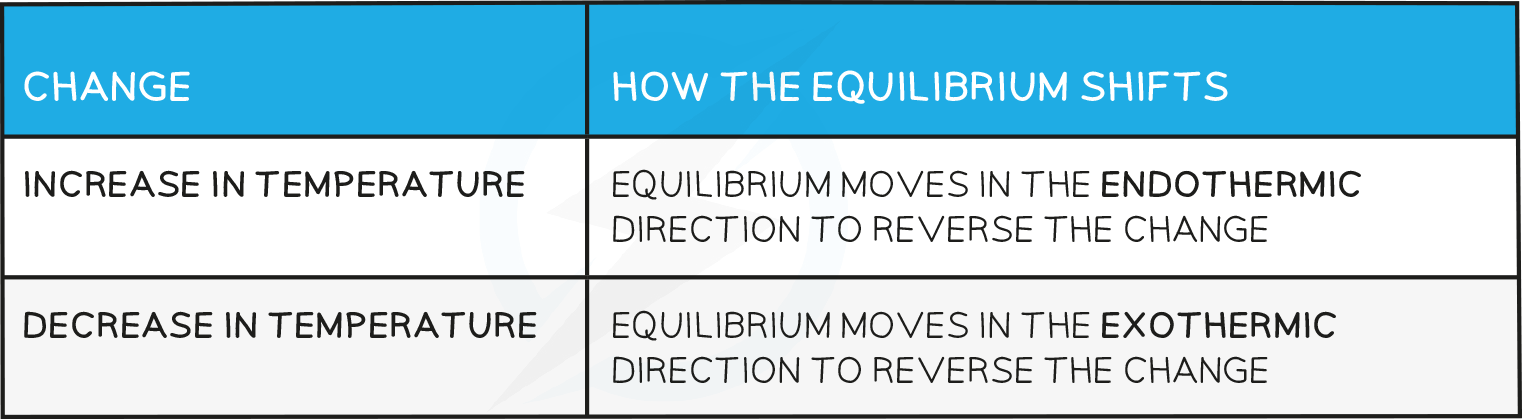
Effects of temperature
- How the equilibrium shifts with temperature changes:
Effect on the value of Kp
- For a reaction that is exothermic in the forward direction, increasing the temperature pushes the equilibrium from right to left
- Therefore, the value of Kp will decrease as the ratio of [ products ] to [ reactants ] decreases
- Conversely, if the temperature is raised in an endothermic reaction, the value of Kp will increase
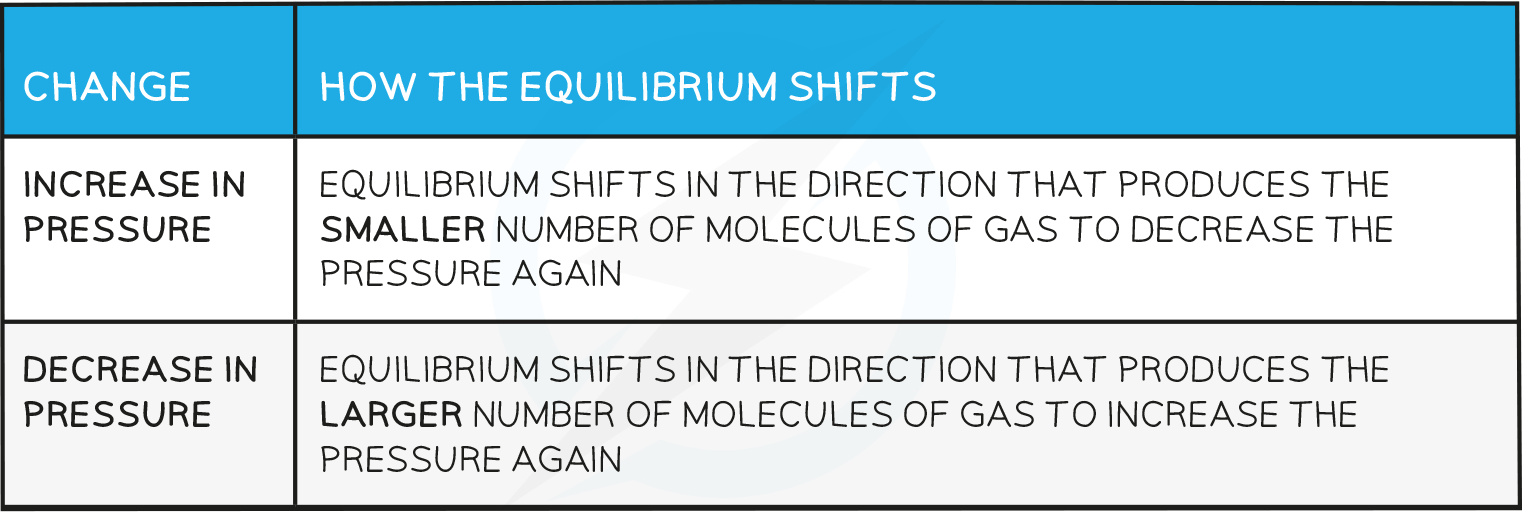
Effects of pressure
- Changes in pressure only affect reactions where the reactants or products are gases
- How the equilibrium shifts with pressure changes:
Effect on the value of Kp
- The value of Kp is not affected by any changes in pressure.
- Changes in pressure cause a shift in the position of equilibrium to a new position which restores the value of Kp
- This is analogous to what happens to Kc when you change concentration in an aqueous equilibrium; a shift restores equilibrium to a new position maintaining Kc
Presence of a catalyst
- If all other conditions stay the same, the equilibrium constant Kp is not affected by the presence of a catalyst
- A catalyst speeds up both the forward and reverse reactions at the same rate so the ratio of [ products ] to [ reactants ] remains unchanged
- Catalysts only cause a reaction to reach equilibrium faster
- Catalysts therefore have no effect on the position of the equilibrium once this is reached
转载自savemyexams

早鸟钜惠!翰林2025暑期班课上线

最新发布
© 2025. All Rights Reserved. 沪ICP备2023009024号-1








