- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
AQA A Level Chemistry复习笔记5.3.2 Partial Pressure
Partial Pressure
- The partial pressure of a gas is the pressure it exerts in a mixture of gases if it occupied the container on its own
- Partial pressure is given the symbol p, so for a gas X, it is written as pX
- The total pressure is the sum of the partial pressures (this is known as Daltons' Law)
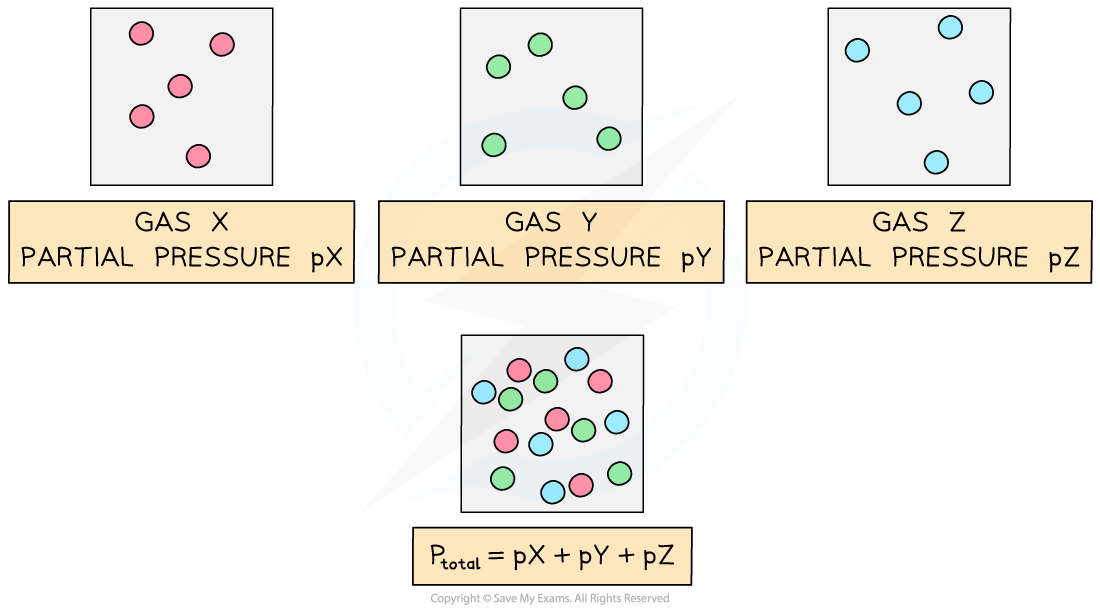
- To find the partial pressure of a gas, you need two pieces of information
- The total pressure in the container
- The mole fraction Kp
- The mathematical relationships are as follows

Partial pressure and mole fraction expressions
Worked Example
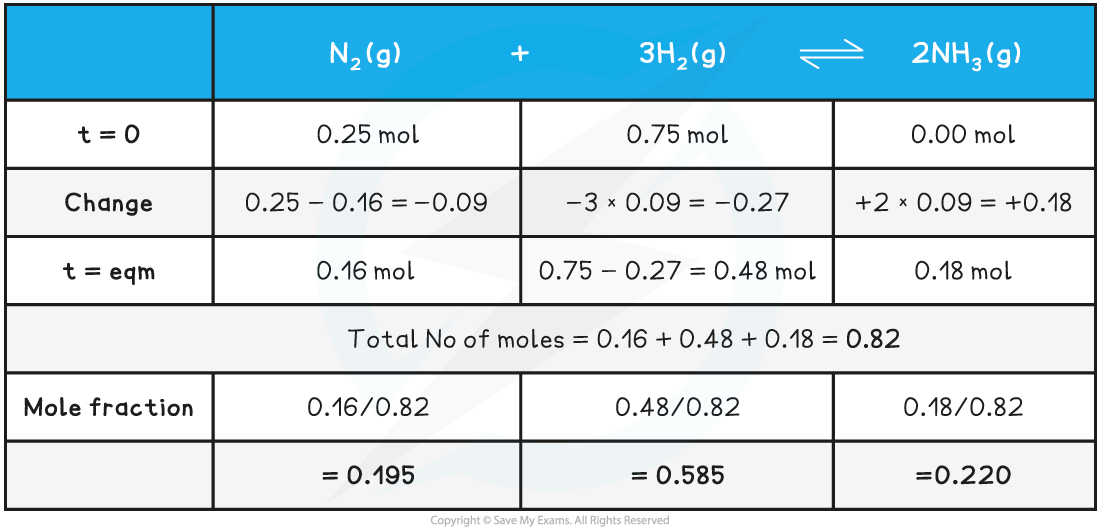
Working out mole fractionsA sample of 0.25 mole of nitrogen and 0.75 mole of hydrogen were reacted together to form ammonia. The equilibrium amount of nitrogen was 0.16 mole.
N2 (g) + 3H2 (g) ⇌ 2NH3 (g)
Calculate the mole fractions of nitrogen, hydrogen and ammonia.
Answer
Write out the equation and record the initial, the change and the equilibrium amounts:
Exam Tip
You can check you have the mole fractions correct by adding them up and making sure they come to 1:0.195 + 0.585 + 0.220 = 1
Kp Calculations
- Kp calculations are a step-by-step process in which you need to find
- the mole fractions of the gases present
- their partial pressures
- the Kp expression
- the value of Kp
- The following worked example shows how this is achieved
Worked Example
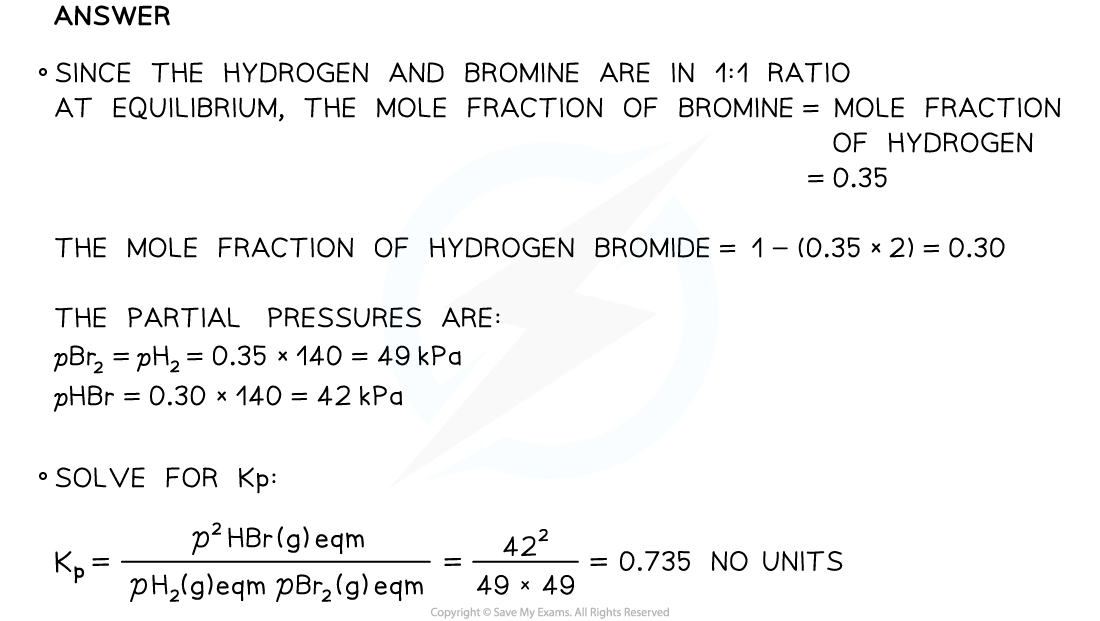
Finding KpHydrogen and bromine were mixed in a flask in a 1: 1 ratio and allowed to reach equilibrium at 450 K. When equilibrium had been achieved the total pressure in the flask was 140 kPa and the mole fraction of bromine was 0.35.The equation for the reaction is
H2 (g) + Br2 (g) ⇌ 2HBr (g)
Determine the partial pressures for each gas at equilibrium and the value of Kp
- Another style of Kp calculation involves being given the value of Kp and working backwards to deduce the partial pressure of one of the gases
Worked Example
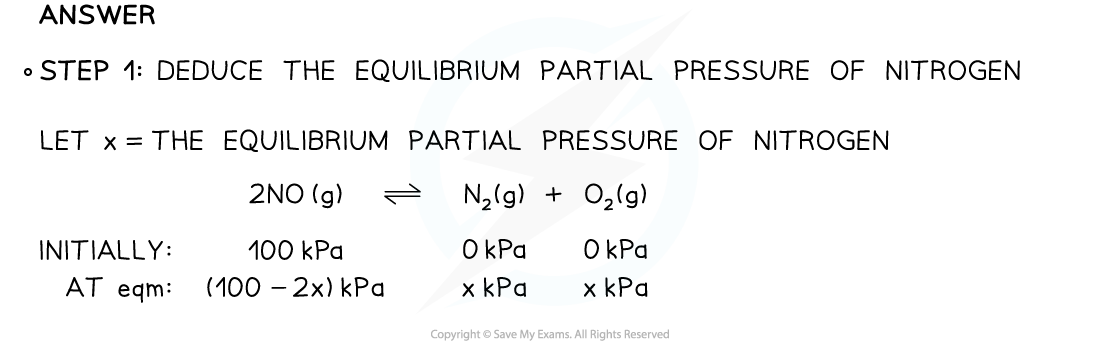
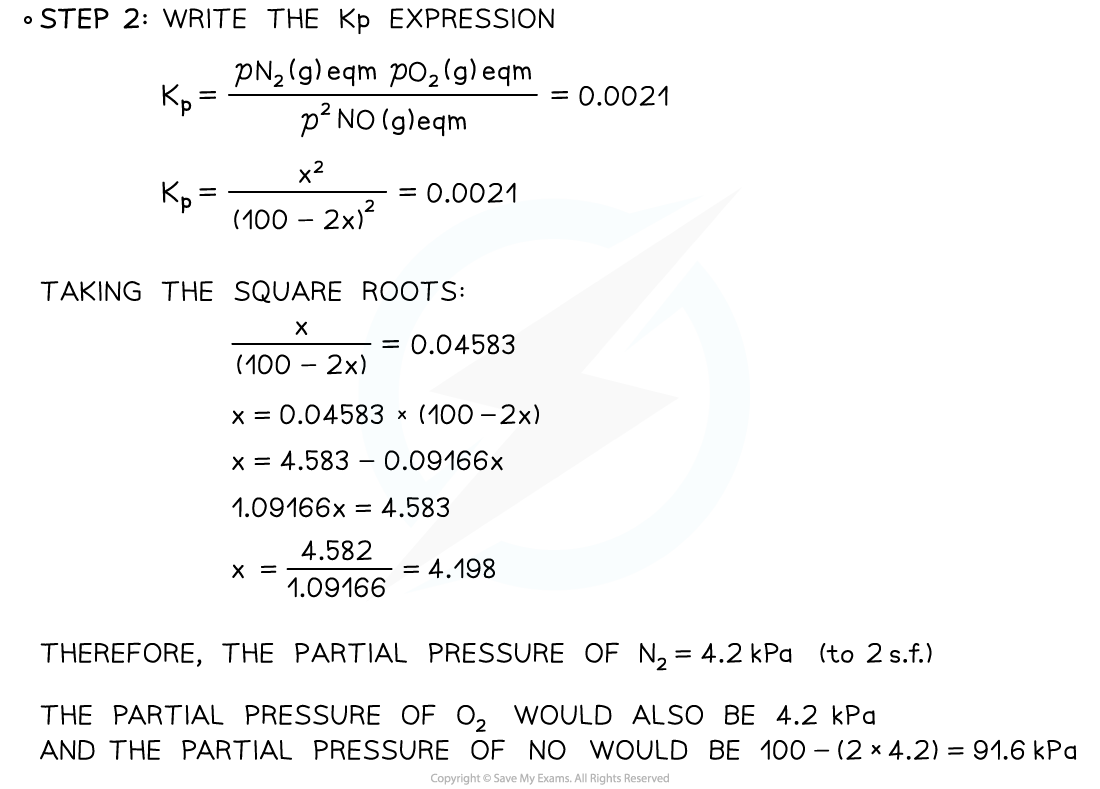
Finding Partial PressureKp for the dissociation equilibrium reaction of nitrogen monoxide is 0.0021.
2NO (g) ⇌ N2 (g) + O2 (g)
If pure NO is introduced into a reaction flask at an initial pressure of 100 kPa, what is the equilibrium partial pressure of nitrogen?
转载自savemyexams

最新发布
© 2025. All Rights Reserved. 沪ICP备2023009024号-1









