- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
AQA A Level Chemistry复习笔记5.2.2 Deriving Rate Equations
Deducing Orders
Order of reaction
- For the general reaction
A + B → C + D
- The order of reaction shows how the concentration of a reactant affects the rate of reaction
Rate = k [A]m [B]n
- When m or n is zero = the concentration of the reactants does not affect the rate
- When the order of reaction (m or n) of a reactant is 0, its concentration is ignored
- The overall order of reaction is the sum of the powers of the reactants in a rate equation
- For example, in the reaction below, the overall order of reaction is 2 (1 + 1)
Rate = k [NO2] [Cl2]
Order of reaction from concentration vs. time graphs
- In a zero-order the concentration of the reactant is inversely proportional to time
- This means that the concentration of the reactant decreases with increasing time
- The graph is a straight line going down
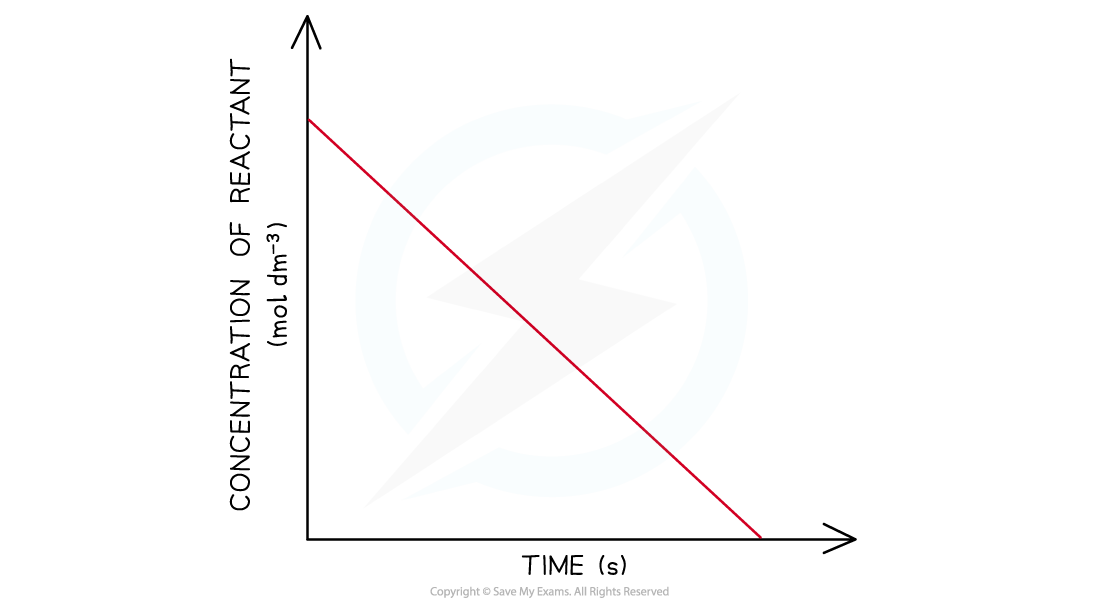
Concentration-time graphs of a zero-order reaction
- In a first-order reaction the concentration of the reactant decreases with time
- The graph is a curve going downwards and eventually plateaus
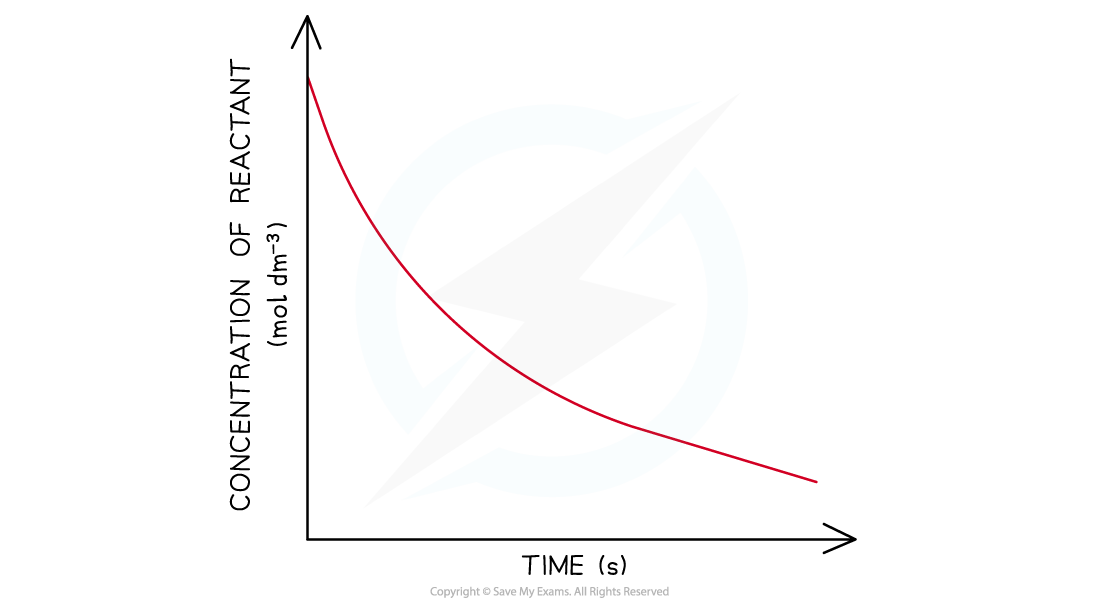
Concentration-time graphs of a first-order reaction
- In a second-order reaction the concentration of the reactant decreases more steeply with time
- The concentration of reactant decreases more with increasing time compared to in a first-order reaction
- The graph is a steeper curve going downwards
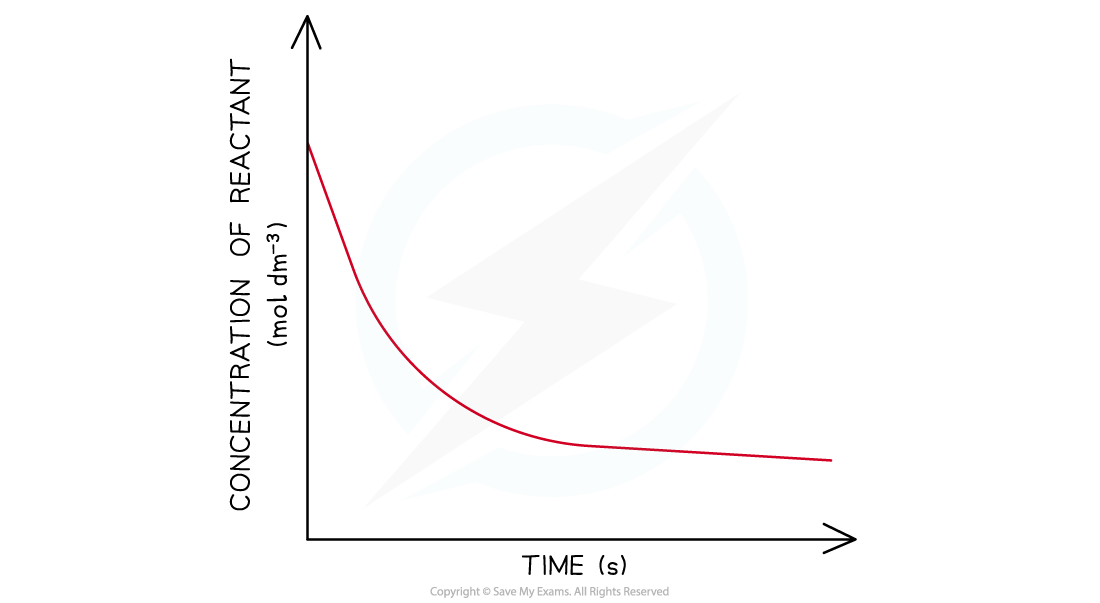
Concentration-time graphs of a second-order reaction
Order of reaction from rate vs. time graphs
- The progress of the reaction can be followed by measuring the initial rates of the reaction using various initial concentrations of each reactant
- These rates can then be plotted against time in a rate-time graph
- In a zero-order reaction the rate doesn’t depend on the concentration of the reactant
- The rate of the reaction therefore remains constant throughout the reaction
- The graph is a horizontal line
- The rate equation for this one reactant is rate = k
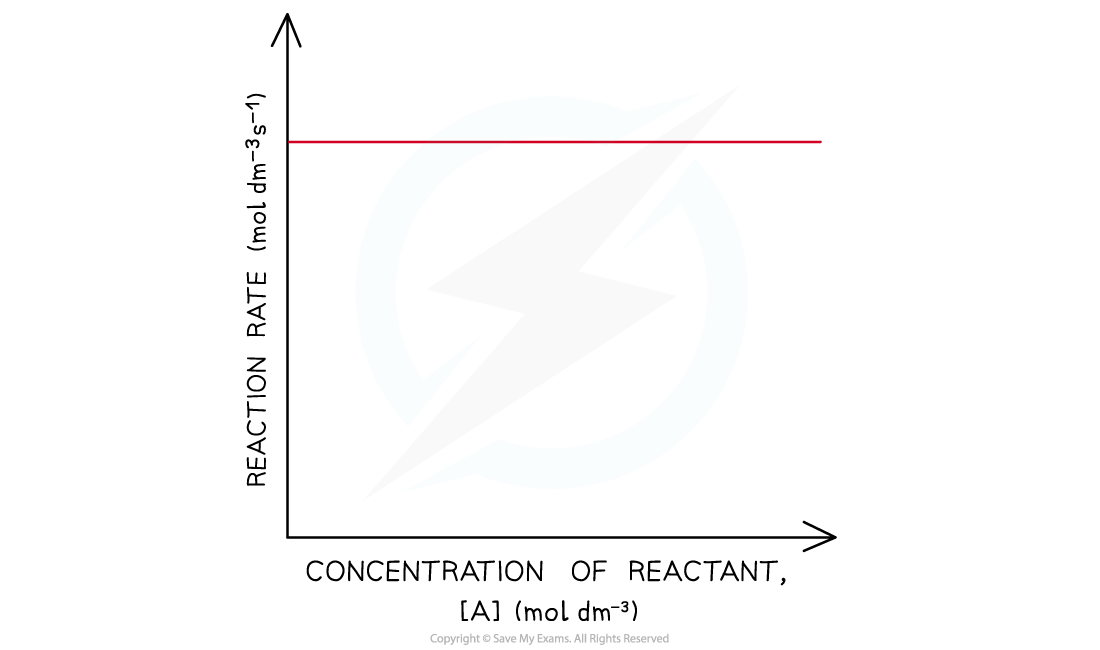
Rate-time graph of a zero-order reaction
- In a first-order reaction the rate is directly proportional to the concentration of a reactant
- This means that if you doubled the concentration of the reactant, the rate would also double
- If you increased the concentration of the reactant by a factor of 3, the rate would increase by this factor as well
- The graph is a straight line
- The rate equation for this one reactant is rate = k [A]
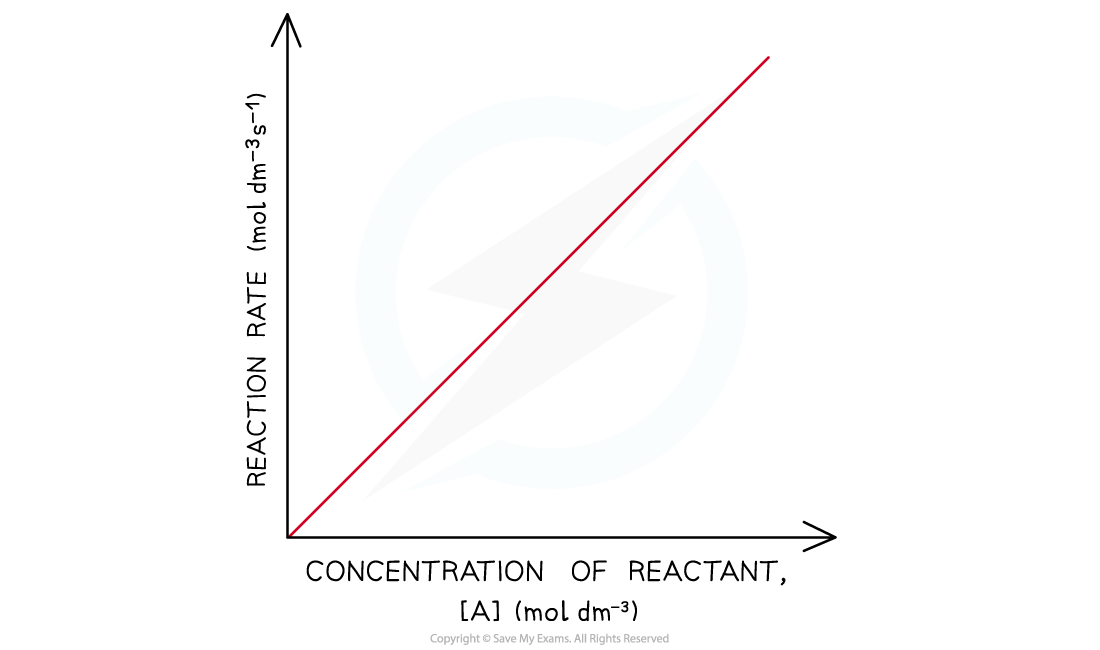
Rate-time graph of a first-order reaction
- In a second-order reaction, the rate is directly proportional to the square of concentration of a reactant
- This means that if you doubled the concentration of the reactant then the rate would increase by 4 (22)
- If you increase the concentration by a factor of 3, then the rate would increase by a factor of 9 (32)
- The graph is a curved line
- The rate equation for this one reactant is rate = k [A]2
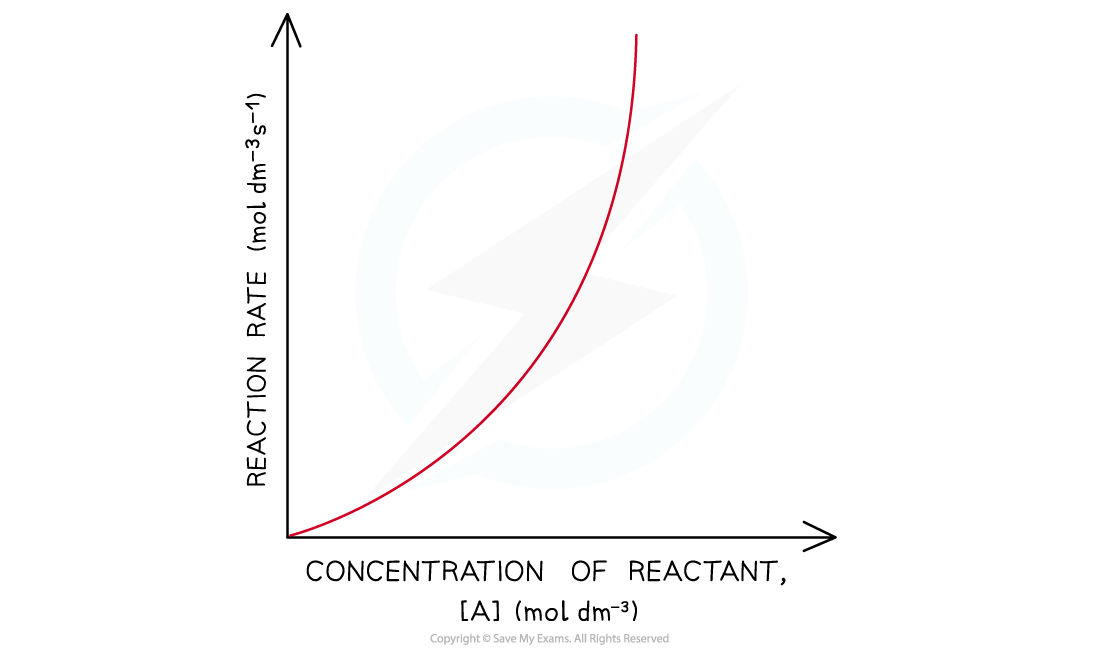
Rate-time graphs of a second-order reaction
Order of reaction from half-life
- The order of a reaction can also be deduced from its half-life (t1/2 )
- The half-life (t1/2) is the time taken for the concentration of a limiting reactant to become half of its initial value
- For a zero-order reaction the successive half-lives decrease with time
- This means that it would take less time for the concentration of reactant to halve as the reaction progresses
- The half-life of a first-order reaction remains constant throughout the reaction
-
-
- The amount of time required for the concentration of reactants to halve will be the same during the entire reaction
-
- For a second-order reaction, the half-life increases with time
-
- This means that as the reaction is taking place, it takes more time for the concentration of reactants to halve

Half-lives of zero, first and second-order reactions
Calculating the initial rate
- The initial rate can be calculated using the initial concentrations of the reactants in the rate equation
- For example, in the reaction of bromomethane (CH3Br) with hydroxide (OH-) ions to form methanol (CH3OH) the reaction equation and rate are as follows:
CH3Br + OH- → CH3OH + Br- (aq)
Rate = k [CH3Br][OH-]
Where k = 1.75 x 10-2 mol-1 dm3 s-1
- If the initial concentrations of CH3Br and OH- are 0.0200 and 0.0100 mol dm-3 respectively, the initial rate of reaction is:
Rate = k [CH3Br] [OH-]
Initial rate = (1.75 x 10-2) x (0.0200) x (0.0100)
Initial rate = 3.50 x 10-6 mol dm-3 s-1
Deriving Rate Equations
Deriving Rate Equations from data
- Let's take the following reaction and derive the rate equation from experimental data
(CH3)3CBr + OH- → (CH3)3COH + Br-
Table to show the experimental data of the above reaction
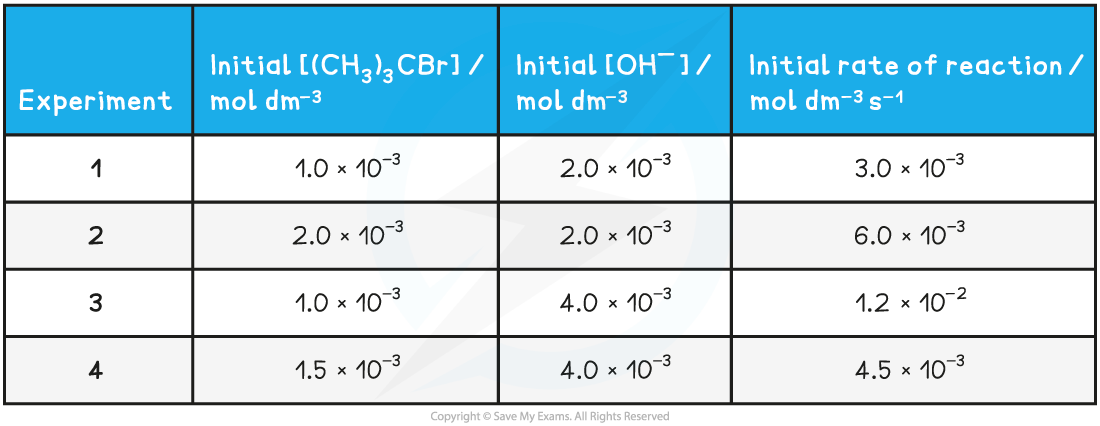
- To derive the rate equation for a reaction, you must first determine all of the orders with respect to each of the reactants
- This can be done using a graph, but it doesn't have to be - you can use tabulated data provided
- Take the reactants one at a time and find the order with respect to each reactant individually
- Identify two experiments where the concentration of the reactant you are looking at first changes, but the concentrations of all other reactants remain constant
- Repeat this for all of the reactants, one at a time, until you have determined the order with respect to all reactants
Order with respect to [(CH3)3CBr]
- From the above table, that is experiments 1 and 2
- The [(CH3)3CBr] has doubled, but the [OH-] has remained the same
- The rate of the reaction has also doubled
- Therefore, the order with respect to [(CH3)3CBr] is 1 (first order)
Order with respect to [OH-]
- From the above table, that is experiments 1 and 3
- The [OH-] has doubled, but the [(CH3)3CBr] has remained the same
- The rate of reaction has increased by a factor of 4 (i.e. increased by 22)
- Therefore, the order with respect to [OH-] is 2 (second order)
Putting the rate equation together
- Once you know the order with respect to all of the reactants, you put them together to form the rate equation
- If a reactant has an order of 0, then you do not include it in the rate equation
- If a reactant has an order of 1, then you do not need to include the number 1 as a power
- If a reactant has an order of 2, then you raise that reactant concentration to the power of 2
- For this reaction, the rate equation will be:
Rate = k [(CH3)3CBr] [OH-]2
Exam Tip
Be careful when reading the values in standard form! It is easy to make a mistake.
转载自savemyexams

最新发布
© 2025. All Rights Reserved. 沪ICP备2023009024号-1









