- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
AQA A Level Chemistry复习笔记4.2.3 Distillation of a Product from a Reaction
Distillation of an Organic Product
REQUIRED PRACTICAL 5
Distillation of an organic product:
- Distillation is a common practical completed in organic chemistry
- For this course, the most common practical in which you will come across distillation and reflux apparatus is the oxidation of primary and secondary alcohol
- This required practical gives you the opportunity to show that:
- You can use an electric heating mantle rather than a Bunsen burner for heating
- Use laboratory apparatus for a variety of experimental techniques and can set up glassware successfully
- Safely and carefully handle different liquids, including those which are corrosive, irritant, flammable and toxic
The Distillation Process:
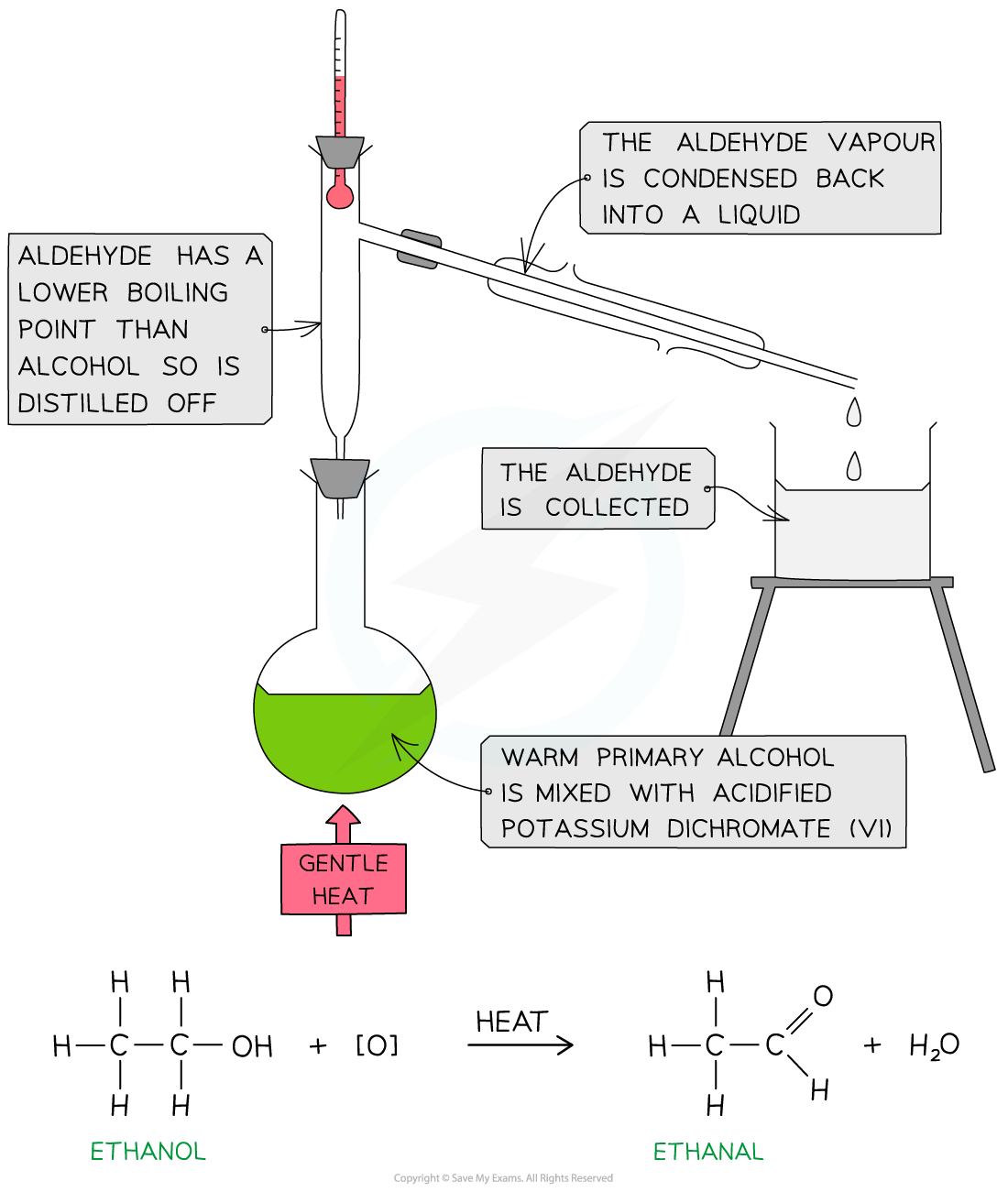
- To produce an aldehyde from a primary alcohol the reaction mixture must be heated
- The aldehyde product has a lower boiling point than the alcohol ( since it has lost the H-bonding) so it can be distilled off as soon as it forms
Heating under Distillation Apparatus
- The reaction mixture needs to be heated until it boils using a heating mantle
- Electric heating mantles are used for this because the temperature can be controlled, and because you are using chemicals which are flammable
- Quickfit apparatus is then set up, including a pear shaped flask, a still head and a condenser
- A Quickfit thermometer can be used, with the thermometer bulb sitting exactly where the vapours will pass into the condenser
- A steady and constant stream of water passes through the condenser in a 'water jacket' - it enters at the bottom of the condenser and the drainage pipe removes the water from the top of the condenser
- The distillate which forms in the condenser drips directly into a receiving vessel
- The distillate which should be collected, is that which is given off at +/- 2 oC of the boiling point of the desired product
- Some distillate may be given off below this temperature - this needs to be discarded and a clean vessel used to collect the desired product
- Stop collecting the distillate if the temperature rises above +/- 2oC of the boiling point of the desired product
转载自savemyexams

早鸟钜惠!翰林2025暑期班课上线

最新发布
© 2025. All Rights Reserved. 沪ICP备2023009024号-1








