- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
AQA A Level Chemistry复习笔记4.1.4 Factors Affecting the Rate of a Reaction
Rate of Reaction
REQUIRED PRACTICAL 3
Factors which affect the rate of a chemical reaction
- During a reaction, the reactants are used up and changed into the products
- The rate of a chemical reaction can be calculated using the following equation:

- As a reaction proceeds, the concentration of the reactants is decreasing and the concentration of the products is increasing
- Because of this, the rate of the reaction is not the same throughout the reaction but changes
- The rate of reaction during the reaction can be calculated from a concentration-time graph
- A tangent will have to be drawn at a certain point on the graph, to calculate the rate at that specific time
- The following graph is an example of cyclopropane being converted to propene
- The concentration of cyclopropene, the reactant, is decreasing over time as the reaction progresses
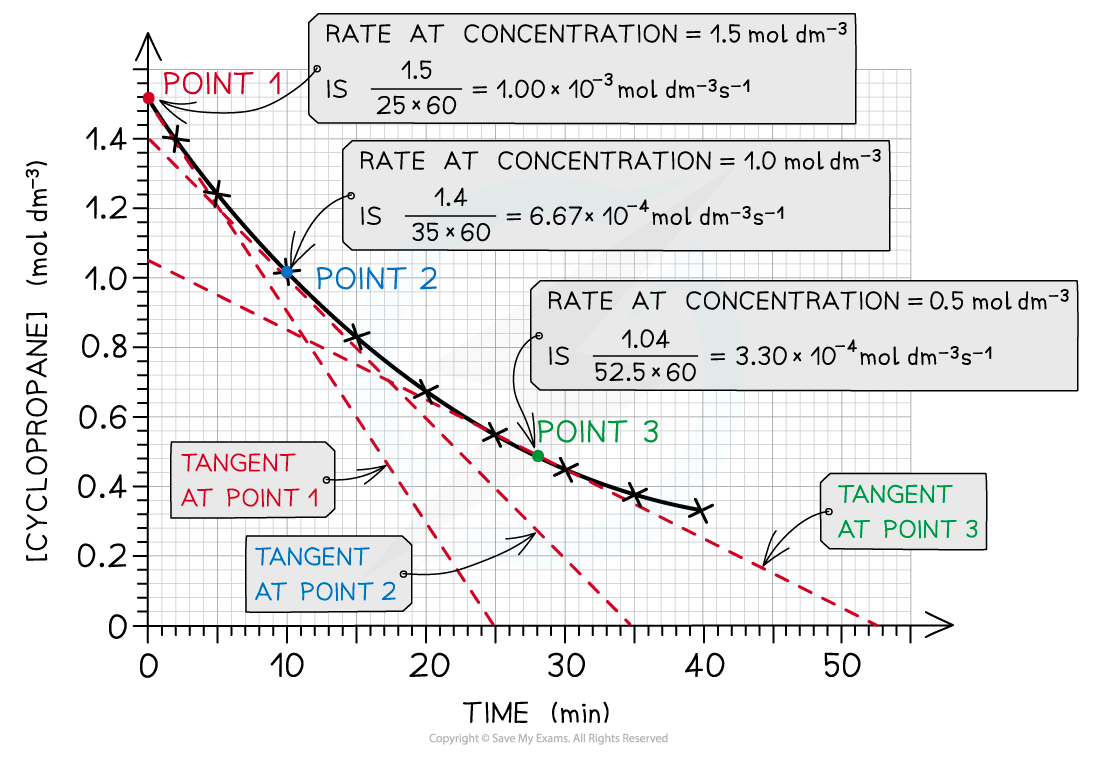
The rate of reaction at 3 different concentrations of cyclopropane is calculated by drawing tangents at those points in the graph
The Disappearing Cross Experiment
- A simple experiment which can be done to determine how rate of reaction is affected by concentration is the disappearing cross experiment
- It is set up as follows:
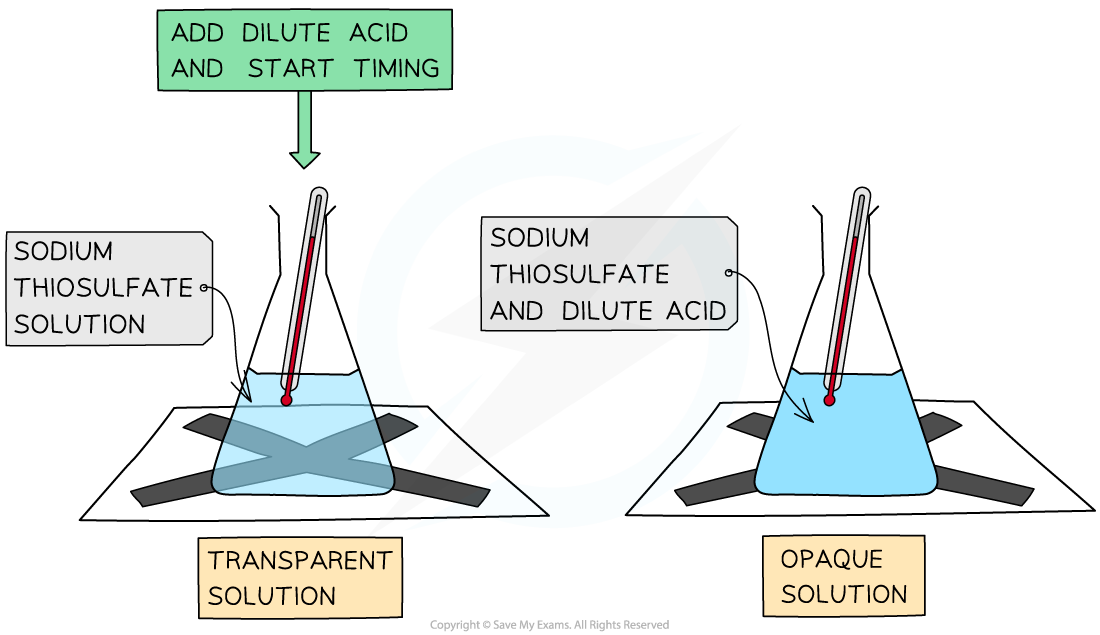
The Disappearing Cross Experiment
- This experiment can be done for a number of different reactions, but taking the following reaction as an example:
Na2S2O3 (aq) + 2HCl (aq) → 2NaCl (aq) + H2O (l) + SO2 (g) + S (s)
- In this reaction, sodium thiosulphate reacts with hydrochloric acid
- The key product which allows this experiment to work, is the sulfur which is a solid, and causes the solution to become opaque
- There are actually two factors which can be investigated using the disappearing cross reaction:
- Changing the temperature
- Changing the concentration of the HCl
- You can plot graphs of concentration against time if the concentration of HCl was changed, or plot 1/t against temperature if the temperature was changed
- You plot 1/t because the rate is proportional to 1/time and the graph you want is rate versus temperature
Exam Tip
The disappearing cross experiment can be used for any reaction where a solid is produced as one of the products, as this will cause the solution to become cloudy. It does not have to be done in a conical flask, it could be done in a test tube with the cross placed underneath the test tube rack. Watch out: this is a very simple experiment, but exam questions could try and make this seem more complicated than it is!
转载自savemyexams


最新发布
© 2025. All Rights Reserved. 沪ICP备2023009024号-1








