- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
AQA A Level Chemistry复习笔记4.1.3 Measurement of an Enthalpy Change
Measurement of an Enthalpy Change
REQUIRED PRACTICAL 2
Measuring enthalpy changes
- Calorimetry is a technique used to measure changes in enthalpy of chemical reactions
- A calorimeter can be made up of a polystyrene drinking cup, a vacuum flask or metal can
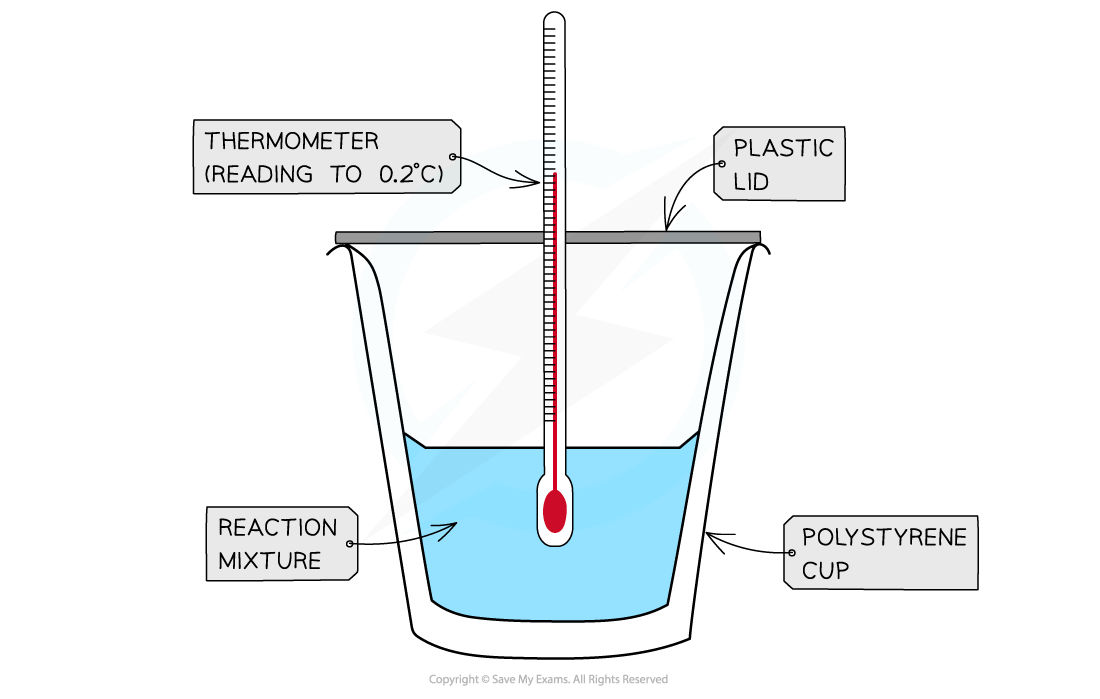
A polystyrene cup can act as a calorimeter to find enthalpy changes in a chemical reaction
- The energy needed to raise the temperature of 1 g of a substance by 1 K is called the specific heat capacity (c) of the liquid
- The specific heat capacity of water is 4.18 J g-1 K-1
- The energy transferred as heat can be calculated by:
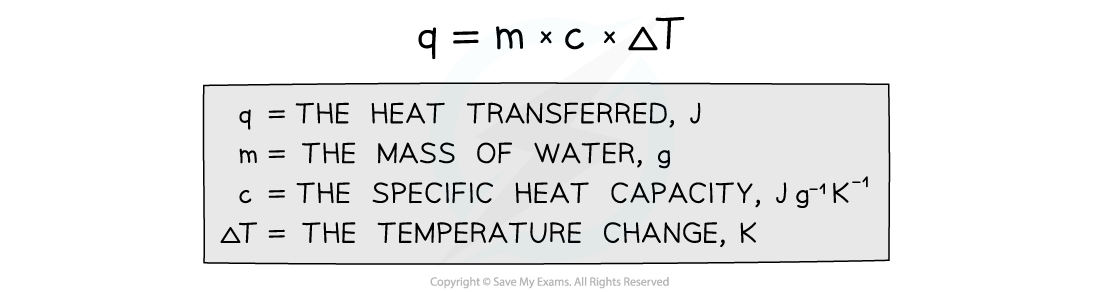
Equation for calculating energy transferred in a calorimeter
- There are two types of calorimetry experiments for you to know:
- Enthalpy changes of reactions in solution
- Enthalpy changes of combustion
- In both cases you should be able to give an outline of the experiment and be able to process experimental data using calculations or graphical methods
Enthalpy changes for reactions in solution
- The principle of these calorimetry experiments is to carry out the reaction with an excess of one reagent and measure the temperature change over the course of a few minutes
- The apparatus needed to carry out an enthalpy of reaction in solution calorimetry experiment is shown above
Sample method for a displacement reaction
- Using a measuring cylinder place 25 cm3 of the 1.0 mol dm-3 copper(II) sulphate solution into the polystyrene cup
- Weigh about 6 g of zinc powder- as this is an excess there is no need to be very accurate
- Draw a table to record the initial temperature and then the temperature and time every half minute up to 9.5 minutes
- Put a thermometer or temperature probe in the cup, stir, and record the temperature every half minute for 2½minutes
- At precisely 3 minutes, add the zinc powder to the cup (DO NOT RECORD THE TEMPERATURE AT 3MIN)
- Continue stirring and record the temperature for an additional 6 minutes
- For the purposes of the calculations, some assumptions are made about the experiment:
- That the specific heat capacity of the solution is the same as pure water, i.e. 4.18 J g-1 K-1
- That the density of the solution is the same as pure water, i.e. 1 g cm-3
- The specific heat capacity of the container is ignored
- The reaction is complete
- There are negligible heat losses
Temperature correction graphs
- For reactions which are not instantaneous there may be a delay before the maximum temperature is reached
- During that delay the substances themselves may be losing heat to the surroundings, so that the true maximum temperature is never actually reached
- To overcome this problem we can use graphical analysis to determine the maximum enthalpy change
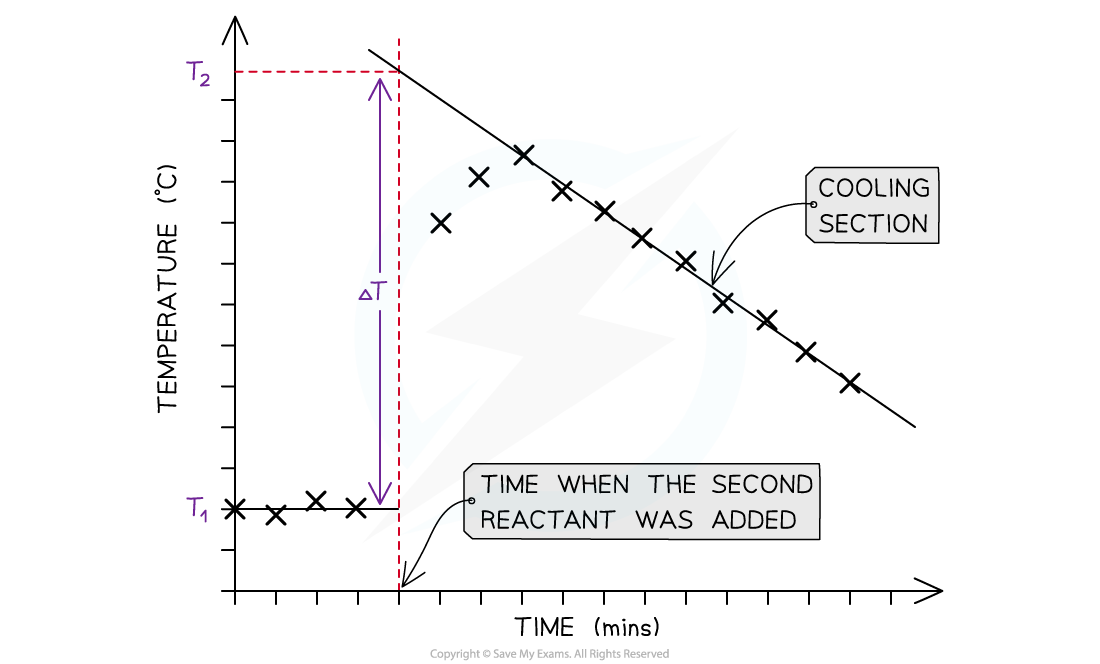
A temperature correction graph for a metal displacement reaction between zinc and copper sulfate solution. The zinc is added after 4 minutes
The steps to make a temperature correction graph are:
- Take a temperature reading before adding the reactants for a few minutes to get a steady value
- Add the second reactant and continue recording the temperature and time
- Plot the graph and extrapolate the cooling part of the graph until you intersect the time at which the second reactant was added
Enthalpy of Combustion Experiments
- The principle here is to use the heat released by a combustion reaction to increase the heat content of water
- A typical simple calorimeter is used to measure the temperature changes to the water
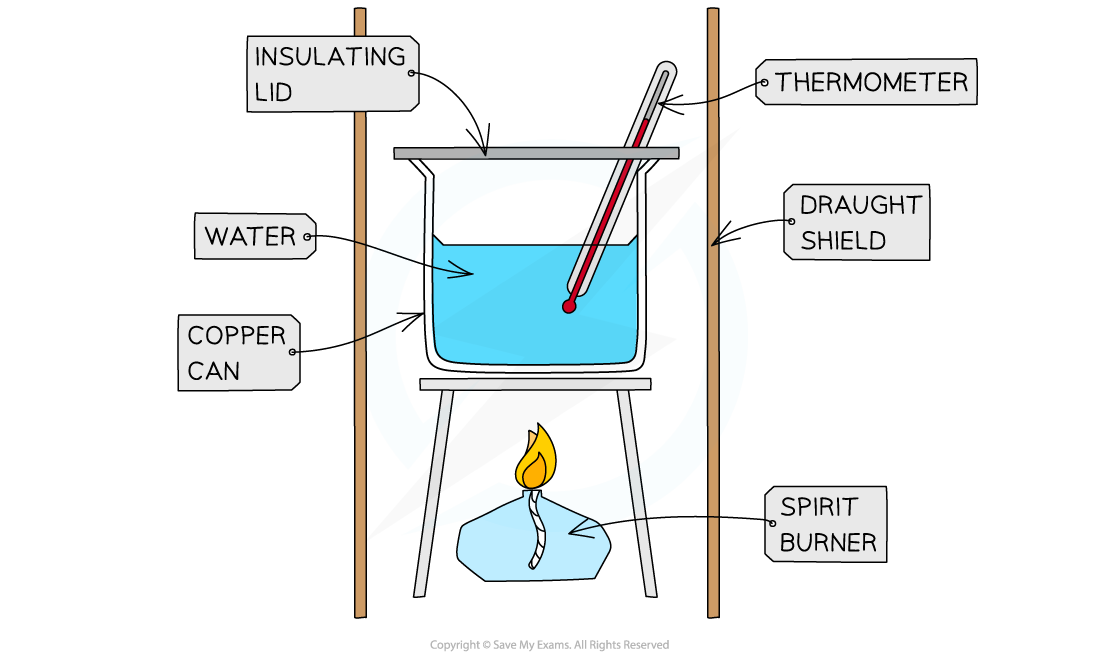
A simple combustion calorimeter
- To complete this experiment, the following steps will need to be completed:
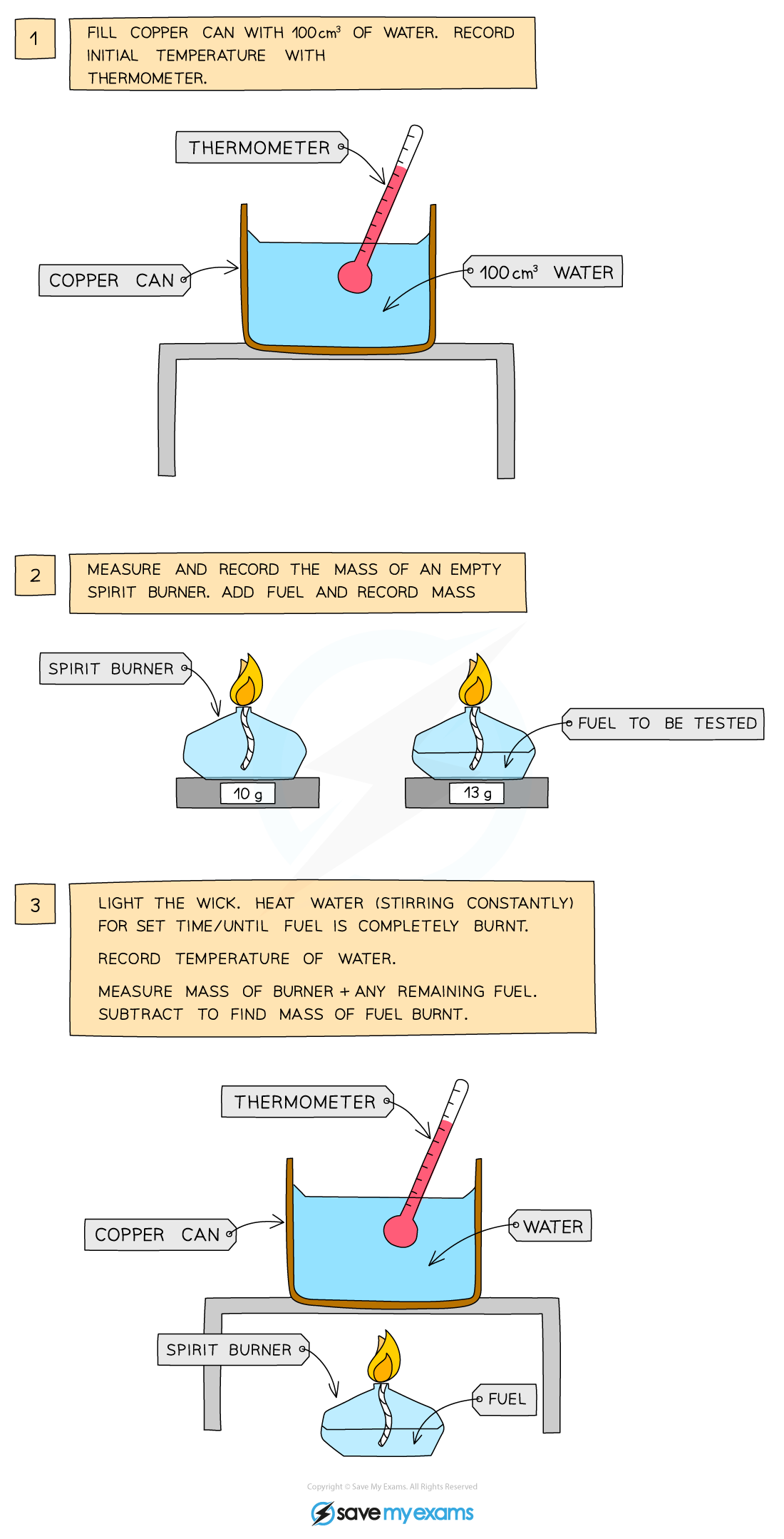
- It is important that you record the starting temperature, and the final temperature in order to complete the calculations
- You must also record the starting mass of the spirit burner and the final mass of the spirit burner, so that you can work out the mass of the fuel burned during the reaction
- This will then be used to calculate the moles, which will be used to convert Q to an enthalpy change in your calculations
Key points to consider
- Not all the heat produced by the combustion reaction is transferred to the water
- Some heat is lost to the surroundings
- Some heat is absorbed by the calorimeter
- To minimise the heat losses the copper calorimeter should not be placed too far above the flame and a lid placed over the calorimeter
- Shielding can be used to reduce draughts
- In this experiment the main sources of error are
- Heat losses
- Incomplete combustion
Worked Example
1.023 g of propan-1-ol (M = 60.11 g mol-1) was burned in a spirit burner and used to heat 200 g of water in a copper calorimeter. The temperature of the water rose by 30 oC. Calculate the enthalpy of combustion of propan-1-ol using this data.
Answer:
Step 1: Calculate q
q = m x c x ΔT
q = 200 g x 4.18 J g-1 K-1 x 30 K = – 25 080 J
Step 2: Calculate the amount of propan-1-ol burned
moles = mass ÷ molar mass = 1.023 g ÷ 60.11 g mol-1 = 0.01702 mol
Step 3: Calculate ΔH
ΔH = q ÷ n = -25 080 J ÷ 0.01702 mol = – 1 473 560 J = -1 474 kJ = -1.5 x 103 kJ
转载自savemyexams

早鸟钜惠!翰林2025暑期班课上线

最新发布
© 2025. All Rights Reserved. 沪ICP备2023009024号-1








