- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
AQA A Level Chemistry复习笔记3.7.3 Nucleophilic Substitution
Mechanism: Nucleophilic Substitution
- A nucleophile is an electron-rich species that can donate a pair of electrons
- ‘Nucleophile’ means ‘nucleus/positive charge loving’ as nucleophiles are attracted to positively charged species
- Nucleophilic refers to reactions that involve a nucleophile
- There are various different species which can behave as nucleophiles, and some make better nucleophiles than others

A hydroxide ion is a better nucleophile as it has a full formal negative charge whereas the oxygen atom in water only carries a partial negative charge
- A nucleophilic substitution reaction is one in which a nucleophile attacks a carbon atom which carries a partial positive charge
- An atom that has a partial negative charge is replaced by the nucleophile
- Halogenoalkanes will undergo nucleophilic substitution reactions due to the polar C-X bond (where X is a halogen)
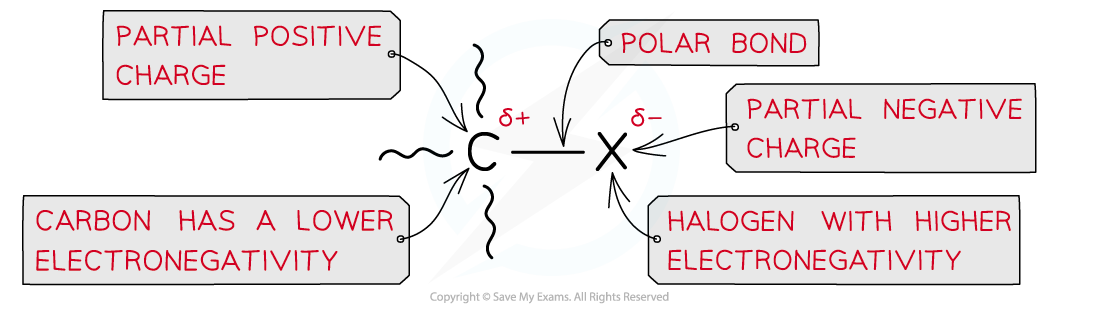
Due to large differences in electronegativity between the carbon and halogen atom, the C-X bond is polar

General Mechanism for Nucleophilic Substitution
- For example, in the following reaction a halogenoalkane reacts with aqueous alkali to form an alcohol

The halogen is replaced by a nucleophile, OH–
- The mechanism for the reaction is as follows

Nucleophilic substitution reaction of bromoethane and aqueous alkali (e.g. NaOH)
Exam Tip
Make sure your arrows are clearly curly - if they are not curly enough then you will not be awarded the mark.Your first arrow must start at the lone pair of the nucleophile and go clearly to the delta positive carbon atom.Your second arrow must start touching the C-X bond and move clearly to the delta negative X atom.Make sure you show the products formed, including the :X- atom which has been substituted.
转载自savemyexams


最新发布
© 2025. All Rights Reserved. 沪ICP备2023009024号-1








