- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
AQA A Level Chemistry复习笔记3.6.2 Mass spectrometry
Interpreting a Mass Spectrum
- Mass spectroscopy is an analytical technique used to identify unknown compounds
- The molecules in the small sample are bombarded with high energy electrons which can cause the molecule to lose an electron
- This results in the formation of a positively charged molecular ion with one unpaired electron
- One of the electrons in the pair has been removed by the beam of electrons

- The molecular ion can further fragment to form new ions, molecules, and radicals
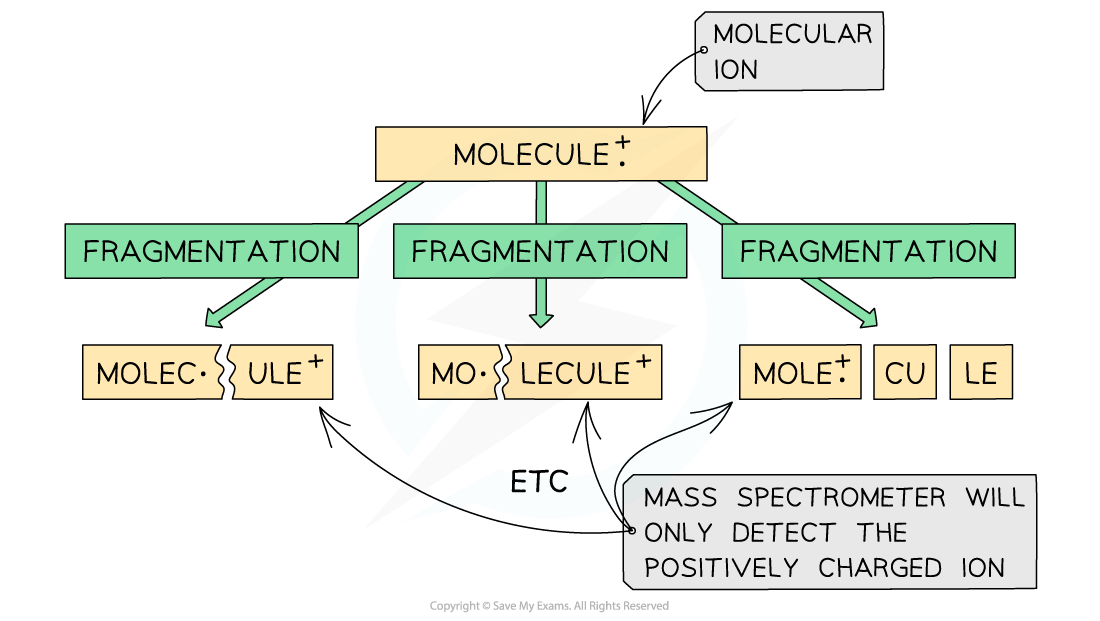
Fragmentation of a molecule in mass spectroscopy
- These fragmented ions are accelerated by an electric field
- Based on their mass (m) to charge (z) ratio, the ion fragments are then separated by deflecting them into the detector
- Most ions will only gain a charge of 1+ and therefore a ion with mass 12 and charge 1+ will have an m/z value of 12
- It is, however, possible for a greater charge to occur. For example, an ion with mass 16 and charge 2+ will have a m/z value of 8
- The smaller and more positively charged fragment ions will be detected first as they will get deflected the most and are more attracted to the negative pole of the magnet
- Each fragment corresponds to a specific peak with a particular m/z value in the mass spectrum
- The base peak is the peak corresponding to the most abundant ion
- The m/z is sometimes referred to as the m/e ratio and it is almost always 1:1
Isotopes
- Isotopes are different atoms of the same element that contain the same number of protons and electrons but a different number of neutrons.
- These are atoms of the same elements but with different mass number
- For example, Cl-35 and Cl-37 are isotopes as they are both atoms of the same element (chlorine, Cl) but have a different mass number (35 and 37 respectively)
- Mass spectroscopy can be used to find the relative abundance of the isotopes experimentally
- The relative abundance of an isotope is the proportion of one particular isotope in a mixture of isotopes found in nature
- For example, the relative abundance of Cl-35 and Cl-37 is 75% and 25% respectively
- This means that in nature, 75% of the chlorine atoms is the Cl-35 isotope and 25% is the Cl-37 isotope
- The heights of the peaks in mass spectroscopy show the proportion of each isotope present
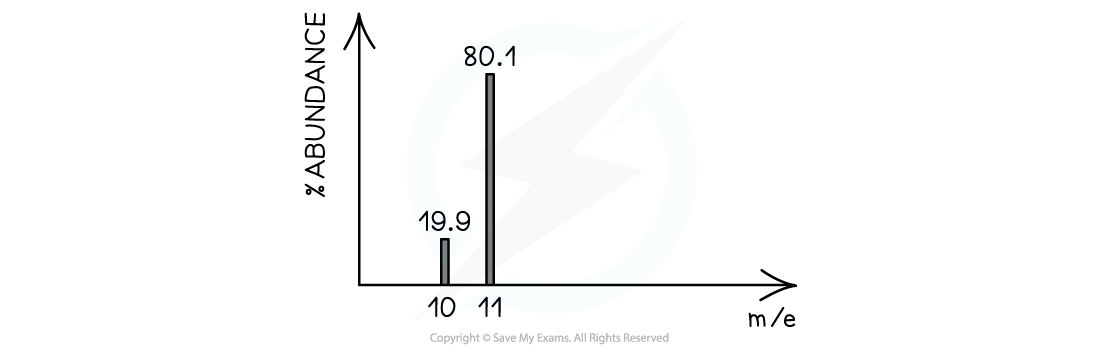
The peak heights show the relative abundance of the boron isotopes: boron-10 has a relative abundance of 19.9% and boron-11 has a relative abundance of 80.1%
Worked Example
Calculating m/z ratio
In a sample of iron, the ions 54Fe2+ and 56Fe3+ are detected. Calculate their m/z ratio and determine which ion is deflected more inside the spectrometer.
Answer
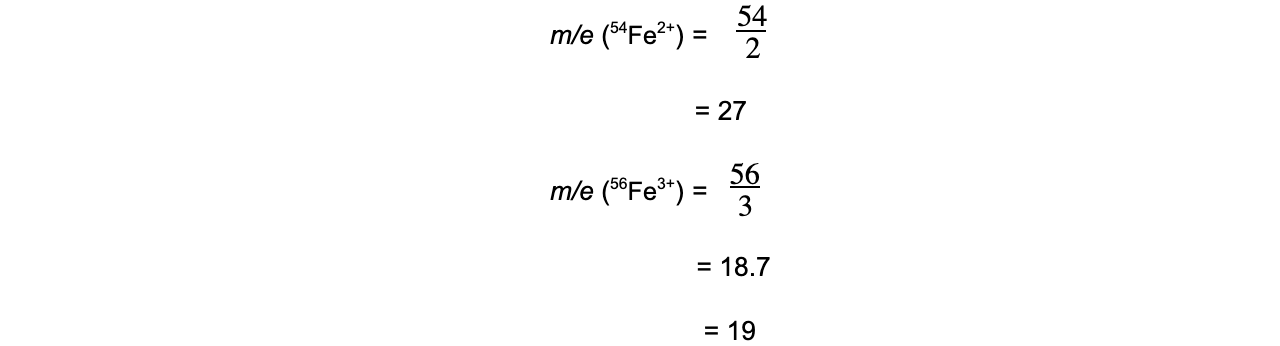
-
- 56Fe3+ has a smaller m/z ratio and will therefore be deflected more.
- It also has the largest positive charge and will be more attracted to the negative pole of the magnet within the mass spectrometer.
Exam Tip
A small m/z value corresponds to fragments that are either small or have a high positive charge or a combination of both.
Deducing Molecular Formula
- Each peak in the mass spectrum corresponds to a certain fragment with a particular m/z value
- The peak with the highest m/z value is the molecular ion (M+) peak which gives information about the molecular mass of the compound
- The molecular ion is the entire molecule that has lost one electron when bombarded with a beam of electrons

- The [M+1] peak is a smaller peak which is due to the natural abundance of the isotope carbon-13
- The amount of naturally occurring C-13 is a little over 1%, so the [M+1] peak is very small
- The height of the [M+1] peak for a particular ion depends on how many carbon atoms are present in that molecule; the more carbon atoms, the larger the [M+1] peak is
- For example, the height of the [M+1] peak for an hexane (containing six carbon atoms) ion will be greater than the height of the [M+1] peak of an ethane (containing two carbon atoms) ion
Worked Example
Analysing mass spectra
Determine whether the following mass spectrum corresponds to but-1-ene or pent-1-ene: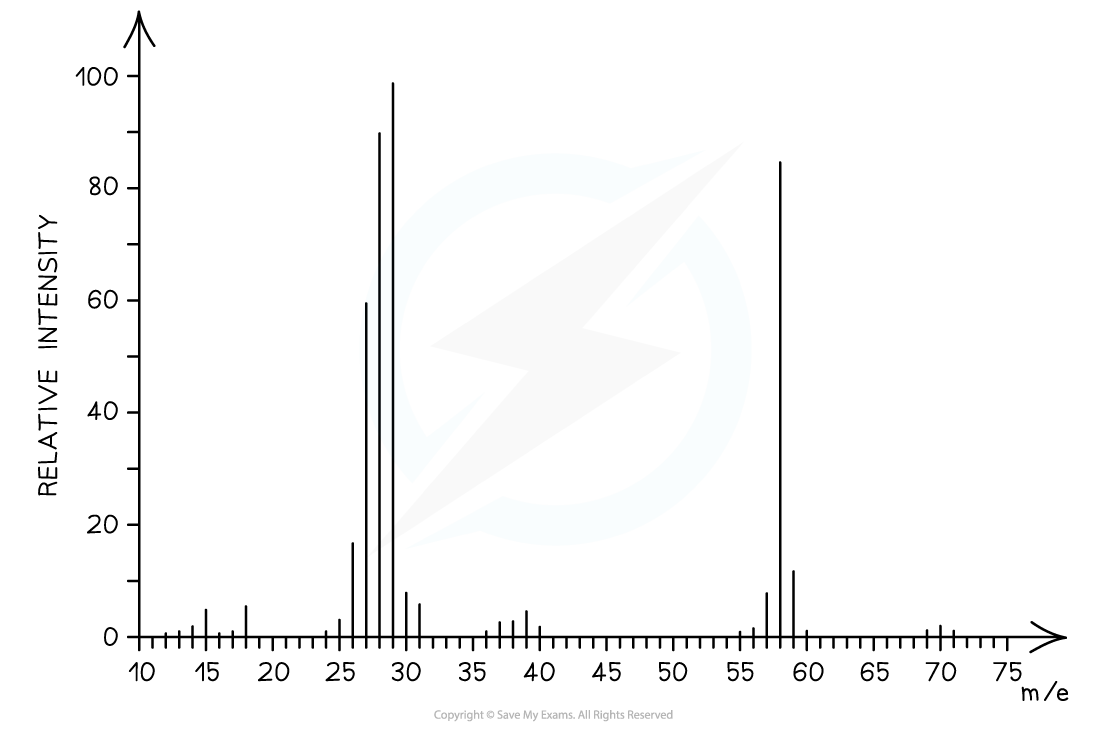
Answer
-
- The mass spectrum corresponds to pent-1-ene as the molecular ion peak is at m/z = 70
- The small peak at m/z = 71 is a C-13 peak, which does not count as the molecular ion peak
- But-1-ene arises from the C4H8+ ion which has a molecular mass of 56
- Pent-1-ene arises from the C5H10+ ion which has a molecular mass of 70
- The mass spectrum corresponds to pent-1-ene as the molecular ion peak is at m/z = 70
Fragmentation
- The molecular ion peak can be used to identify the molecular mass of a compound
- However, different compounds may have the same molecular mass
- To further determine the structure of the unknown compound, fragmentation is used
- Fragments may appear due to the formation of characteristic fragments or the loss of small molecules
- For example, a peak at 29 is due to the characteristic fragment C2H5+
- Loss of small molecules give rise to peaks at 18 (H2O), 28 (CO), and 44 (CO2)
Alkanes
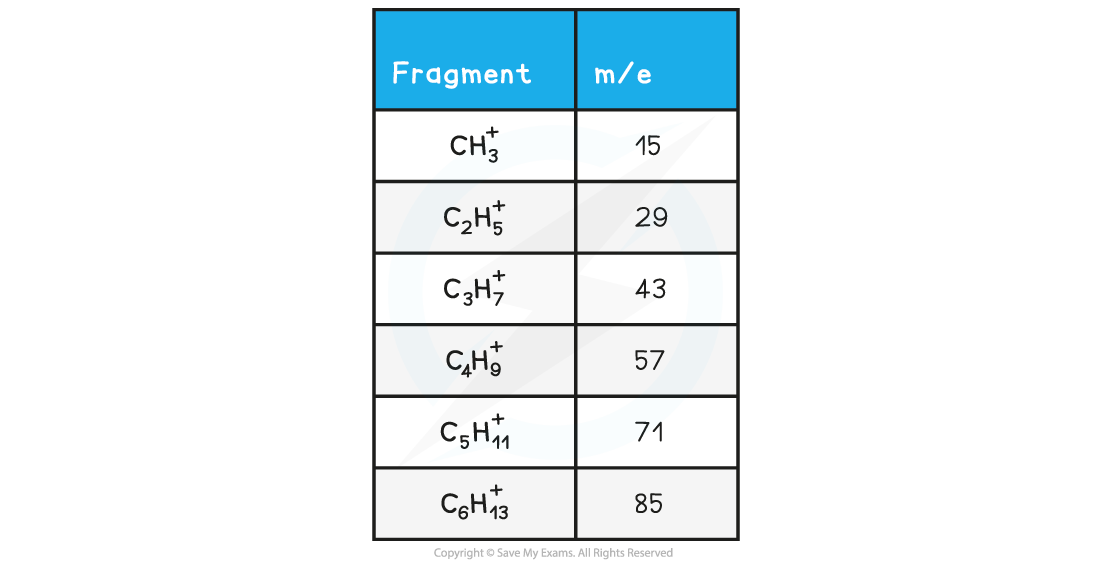
- Simple alkanes are fragmented in mass spectroscopy by breaking the C-C bonds
- M/e values of some of the common alkane fragments are given in the table below
m/e Values of Fragments Table
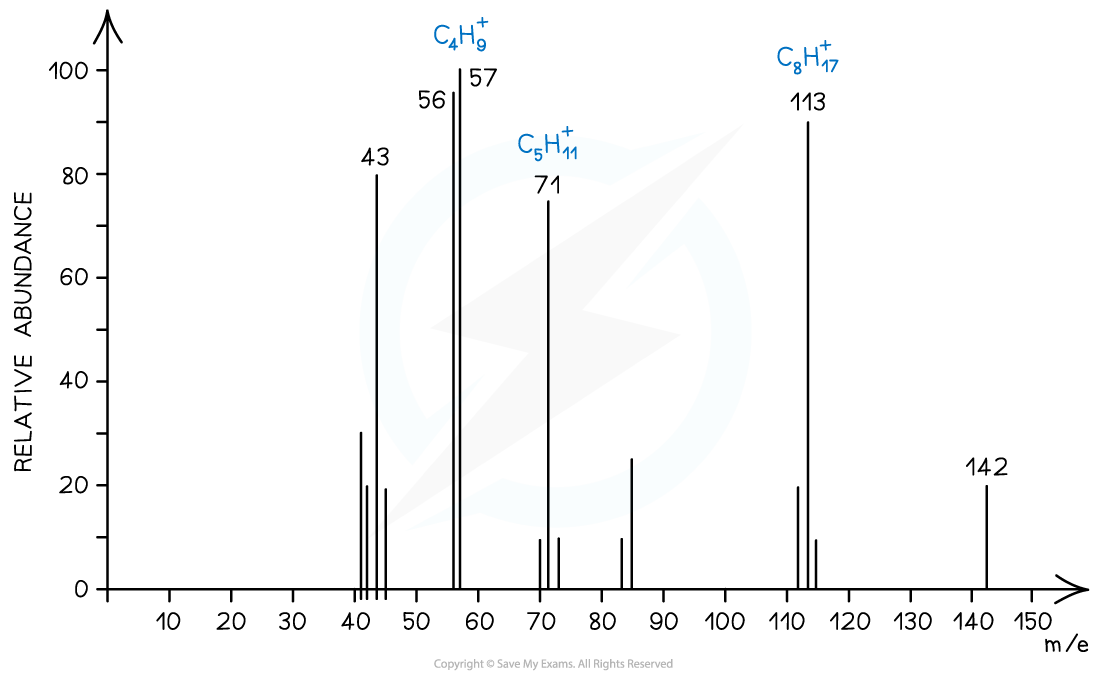
Mass spectrum showing the fragmentation of C10H22
Halogenoalkanes
- Halogenoalkanes often have multiple peaks around the molecular ion peak
- This is caused by the fact that there are different isotopes of the halogens
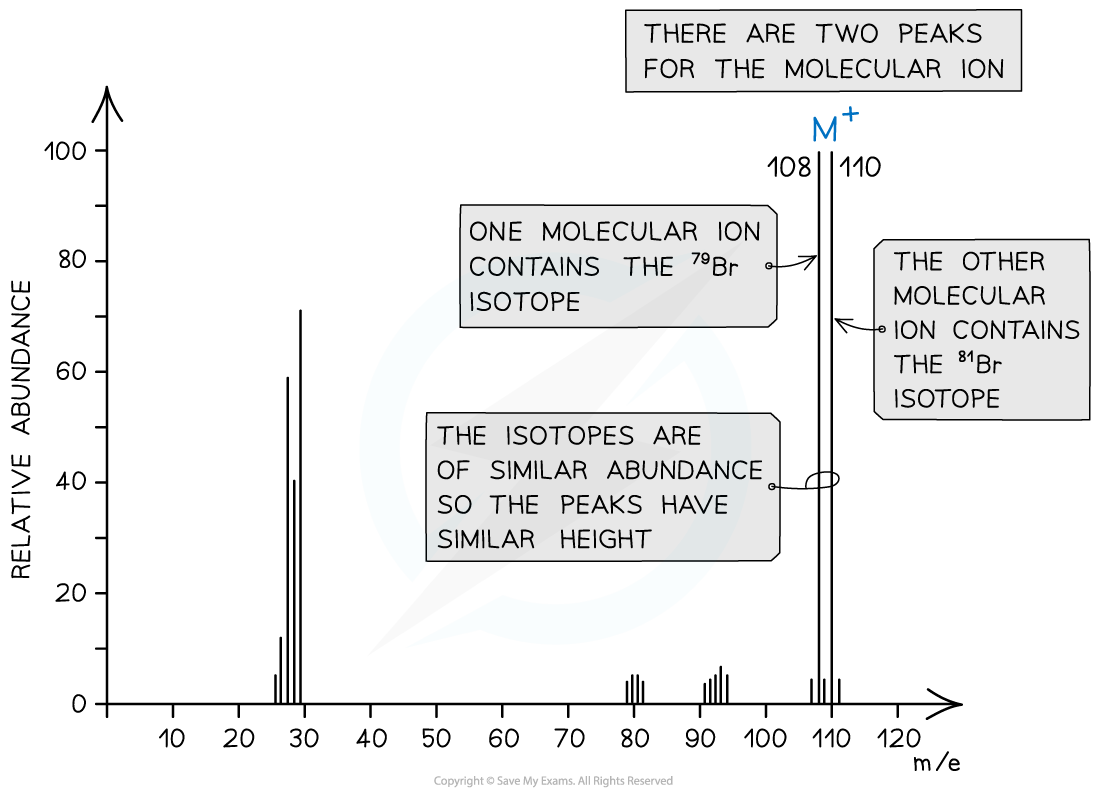
Mass spectrum showing different isotopes of bromine in the molecular ion
Alcohols
- Alcohols often tend to lose a water molecule giving rise to a peak at 18 below the molecular ion
- Another common peak is found at m/e value 31 which corresponds to the CH2OH+ fragment
- For example, the mass spectrum of propan-1-ol shows that the compound has fragmented in four different ways:
- Loss of H• to form a C3H7O+ fragment with m/e = 59
- Loss of a water molecule to form a C3H6+ fragment with m/e = 42
- Loss of a •C2H5 to form a CH2OH+ fragment with m/e = 31
- And the loss of •CH2OH to form a C2H5+ fragment with m/e = 29
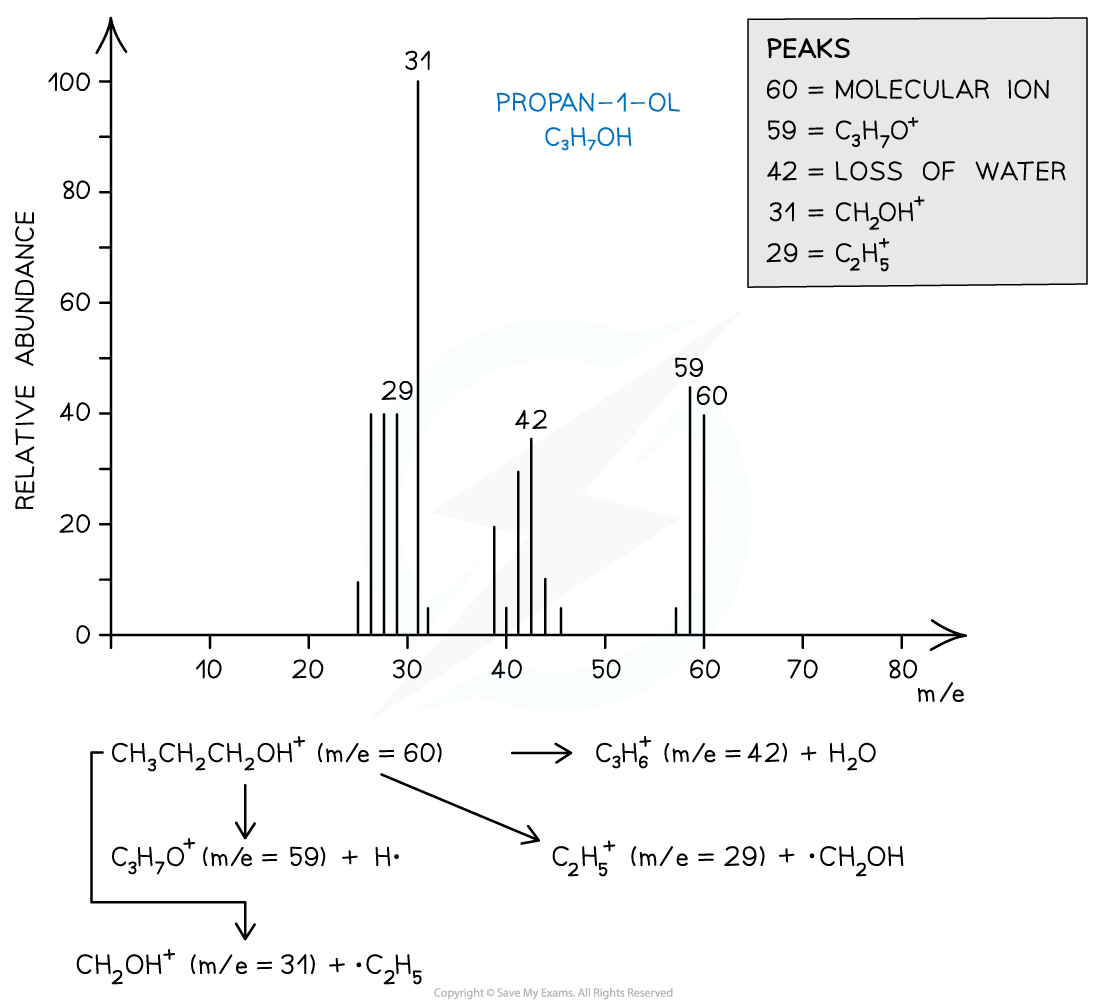
Mass spectrum showing the fragmentation patterns in propan-1-ol (alcohol)
Worked Example
Ion fragmentation
Which of the following statements about the mass spectrum of CH3Br is correct?
A. There is one peak for the molecular ion with an m/e value of 44
B. There is one peak for the molecular ion with an m/e value of 95
C. The last two peaks have abundances in the ratio 3:1 and occur at m/e values of 94 and 96
D. The last two peaks are of equal size and occur at m/e values of 94 and 96
Answer
The correct answer is option D
-
- Bromomethane (CH3Br) can produce 3 peaks
- CH381Br → [CH381Br]+ + e− at m/e 96
- CH379Br → [CH379Br]+ + e− at m/e 94
- CH3Br → [CH3]+ + •Br at m/e 15
- The last two peaks (which correspond to the molecular ion peak) therefore are equal in size and occur at m/e values of 94 and 96
- Bromomethane (CH3Br) can produce 3 peaks
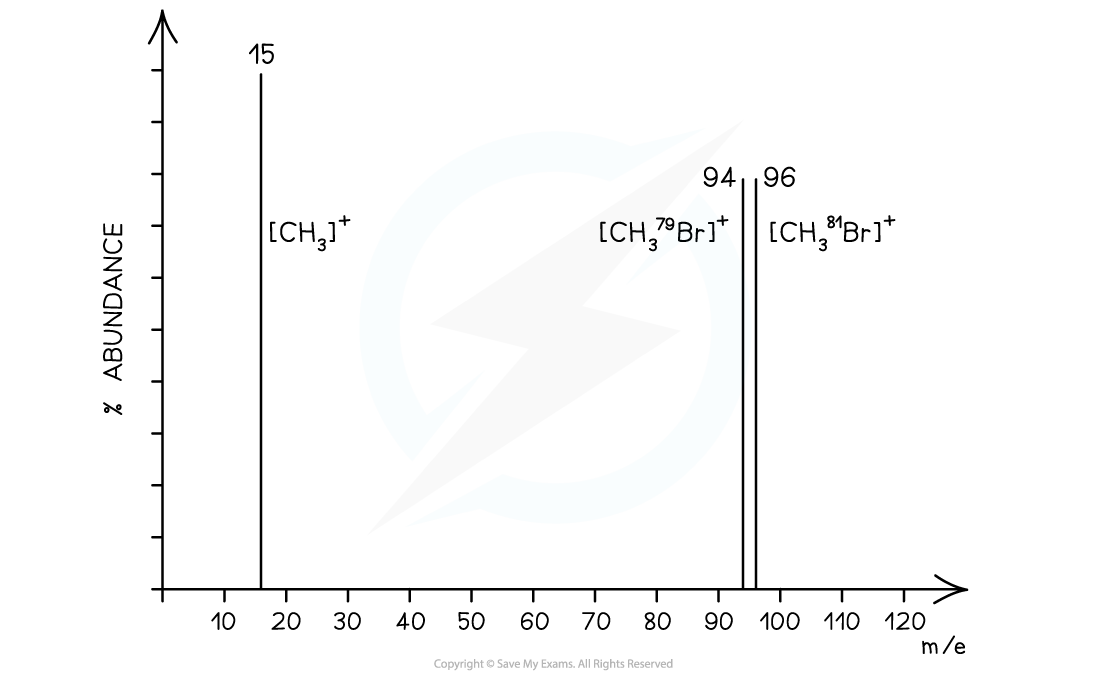
Worked Example
Alcohol fragmentation
Which alcohol is not likely to have a fragment ion at m/e at 43 in its mass spectrum?
A. (CH3)2CHCH2OH
B. CH3CH(OH)CH2CH2CH3
C. CH3CH2CH2CH2OH
D. CH3CH2CH(OH)CH3
Answer
The correct answer is option D
-
- Because a line at m/e = 43 corresponds to an ion with a mass of 43 for example:
- [CH3CH2CH2]+
- [(CH3)2CH]+
- 2-butanol is not likely to have a fragment at m/e = 43 as it does not have either of these fragments in its structure.
- Because a line at m/e = 43 corresponds to an ion with a mass of 43 for example:
转载自savemyexams

最新发布
© 2025. All Rights Reserved. 沪ICP备2023009024号-1









