- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
AQA A Level Chemistry复习笔记3.5.3 Oxidation of Alcohols
Oxidation of Alcohols
Oxidation of alcohols
- Primary alcohols can be oxidised to form aldehydes which can undergo further oxidation to form carboxylic acids
- Secondary alcohols can be oxidised to form ketones only
- Tertiary alcohols do not undergo oxidation
- The oxidising agents of alcohols include acidified K2Cr2O7 or acidified KMnO4
- Acidified potassium dichromate(VI), K2Cr2O7, is an orange oxidising agent
- Acidified means that that the potassium dichromate(VI) is in a solution of dilute acid (such as dilute sulfuric acid)
- For potassium dichromate(VI) to act as an oxidising agent, it itself needs to be reduced
- This reduction requires hydrogen (H+) ions which are provided by the acidic medium
- When alcohols are oxidised the orange dichromate ions (Cr2O72-) are reduced to green Cr3+ ions
- Acidified potassium manganate(VII), KMnO4, is a purple oxidising agent
- As with acidified K2Cr2O7 the potassium manganate(VII) is in an acidic medium to allow reduction of potassium manganate(VII) to take place
- When alcohols are oxidised, the purple manganate ions (MnO4-) are reduced to colourless Mn2+ ions
- As with acidified K2Cr2O7 the potassium manganate(VII) is in an acidic medium to allow reduction of potassium manganate(VII) to take place
- The primary alcohol is added to the oxidising agent and warmed
- The aldehyde product has a lower boiling point than the alcohol reactant so it can be distilled off as soon as it forms
- If the aldehyde is not distilled off, further refluxing with excess oxidising agent will oxidise it to a carboxylic acid
- Since ketones cannot be further oxidised, the ketone product does not need to be distilled off straight away after it has been formed
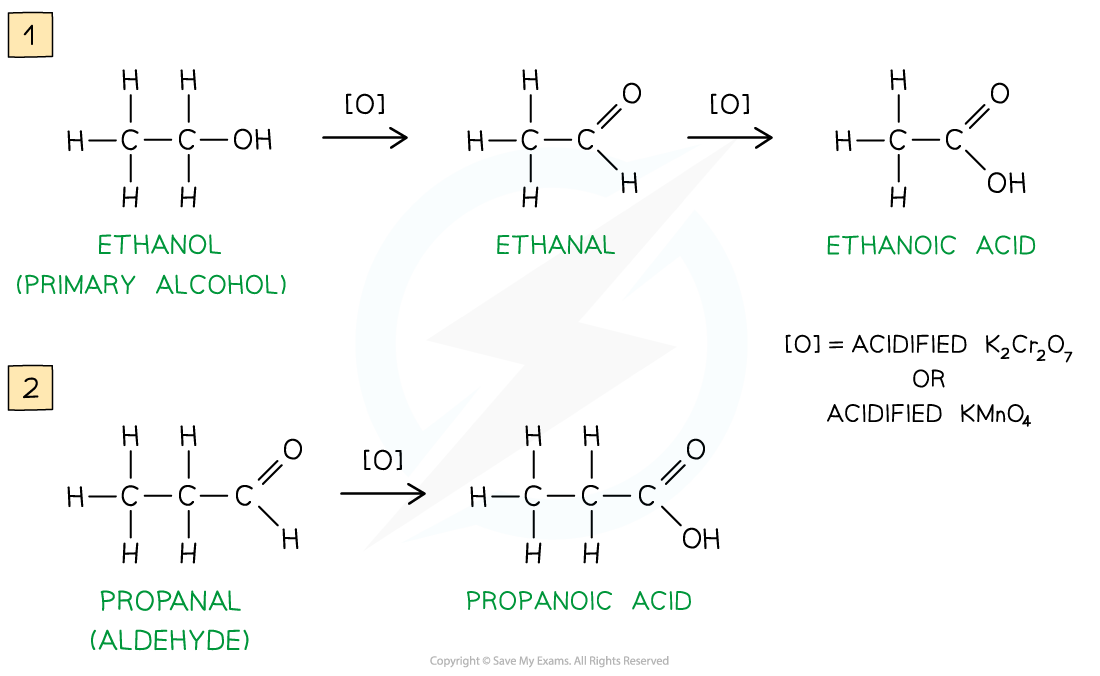
Oxidation Stages of Primary Alcohols
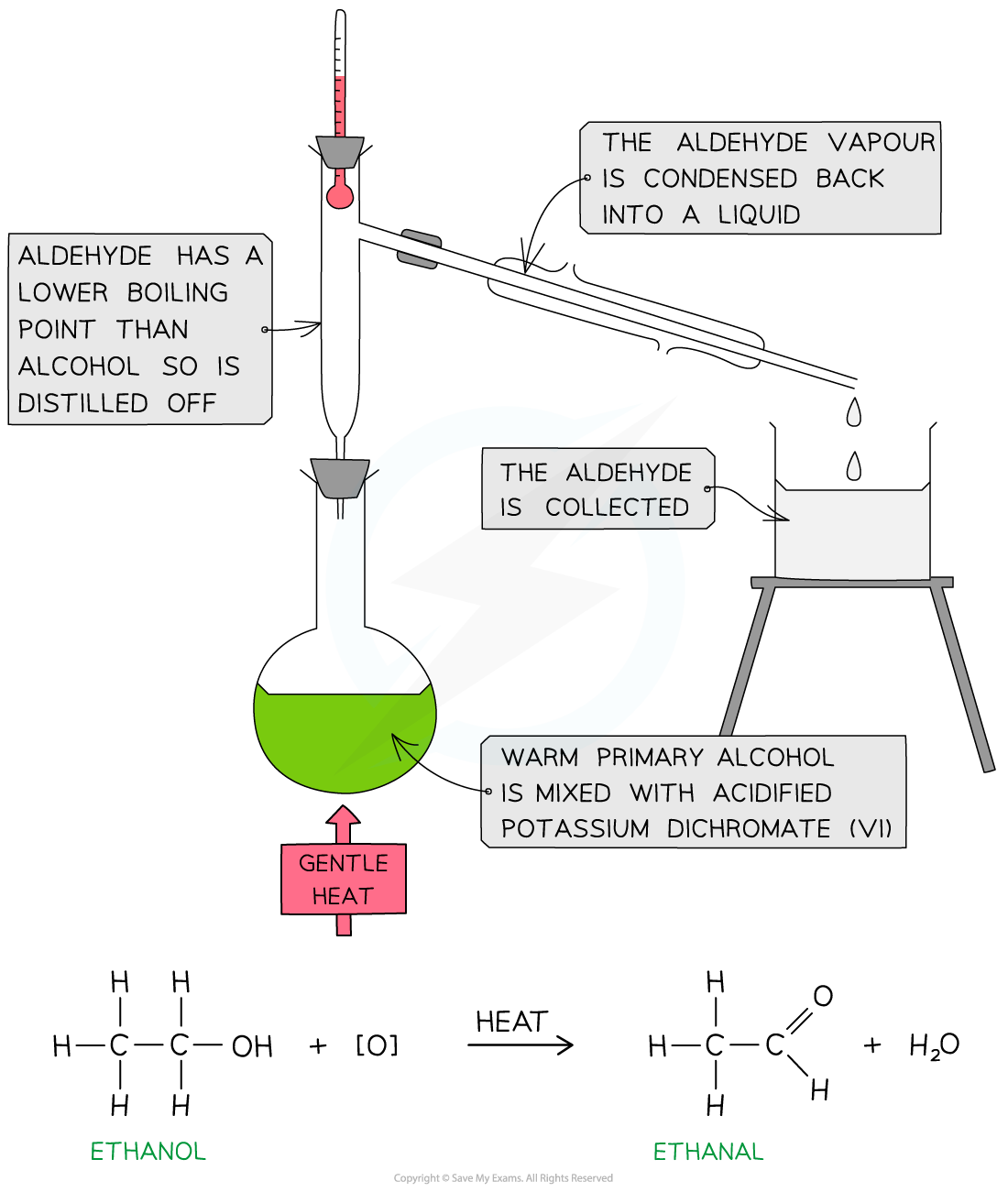
Oxidation of ethanol by acidified K2Cr2O7 to form an aldehyde by distillation
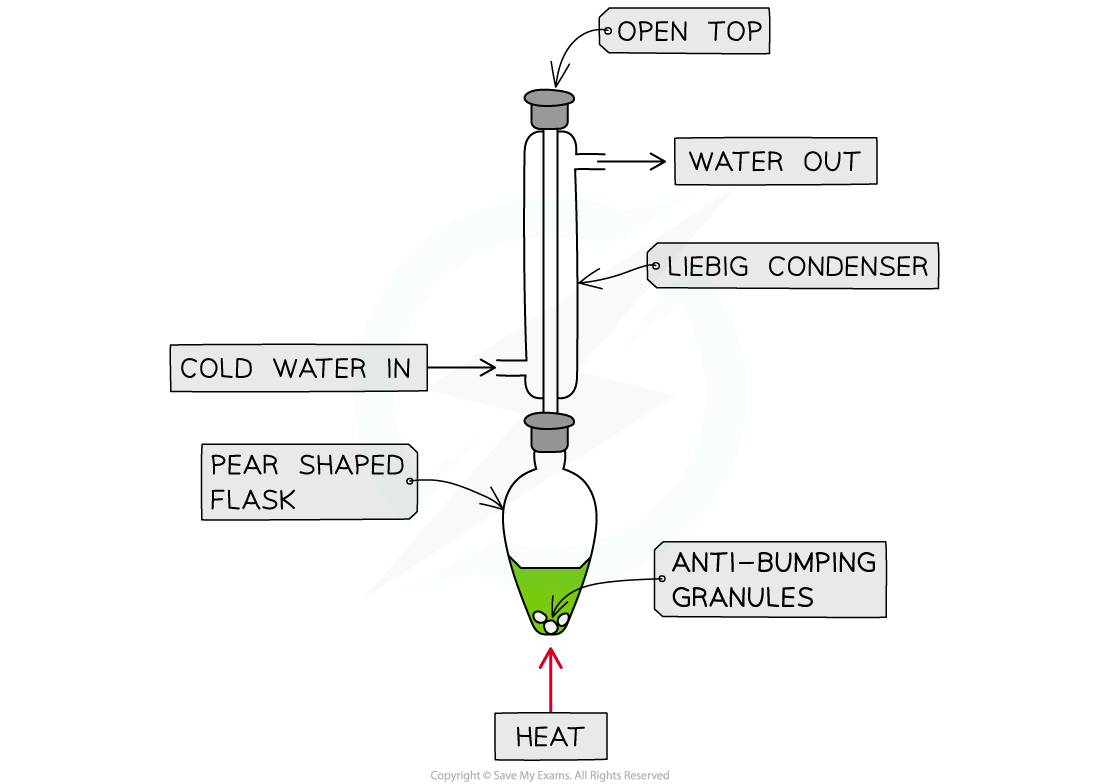
Further oxidation of the aldehyde via reflux can be done to produce a carboxylic acid

Oxidation of propan-2-ol by acidified K2Cr2O7 to form a ketone
Oxidation Products
- Aldehydes and ketones are carbonyl compounds containing a C=O group
- They can be prepared from the oxidation of primary and secondary alcohols respectively
Oxidising agents
- The oxidising agents used to prepare aldehydes and ketones from alcohols include acidified potassium dichromate (K2Cr2O7) and acidified potassium manganate (KMnO4)
- The acidified potassium dichromate(VI), K2Cr2O7, is an orange oxidising agent
- When the alcohols are oxidised the orange dichromate ions (Cr2O72-) are reduced to green Cr3+ ions
- The acidified potassium manganate(VII), KMnO4 is a purple oxidising agent
- When the alcohols are oxidised the purple manganate ions (MnO4-) are reduced to colourless Mn2+ ions
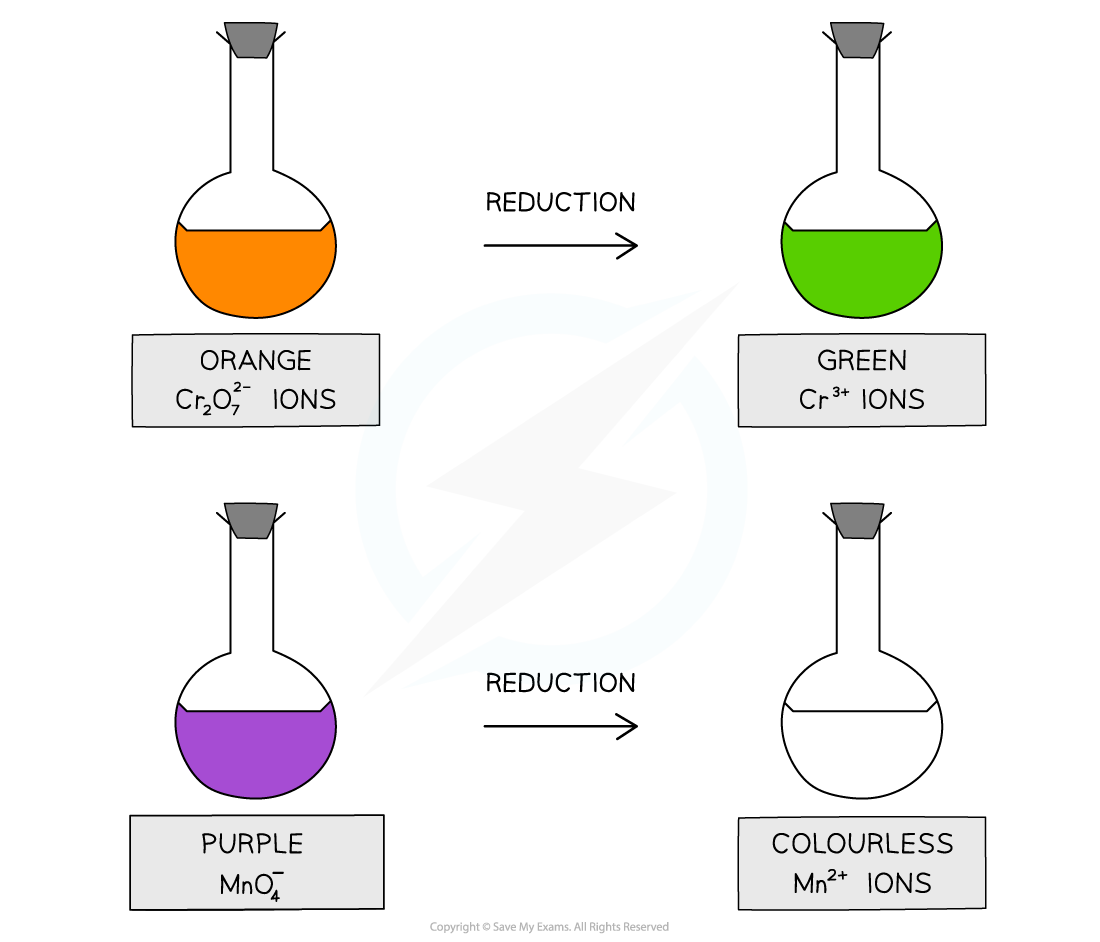
The oxidising agents change colour when they oxidise an alcohol and get reduced themselves
Testing for Oxidation Products
- The presence of an aldehyde group (-CHO) in an unknown compound can be determined by the oxidising agents Fehling’s and Tollens’ reagents
Fehling’s solution
- Fehling’s solution is an alkaline solution containing copper(II) ions which act as the oxidising agent
- When warmed with an aldehyde, the aldehyde is oxidised to a carboxylic acid and the Cu2+ ions are reduced to Cu+ ions
- In the alkaline conditions, the carboxylic acid formed will be neutralised to a carboxylate ion (the -COOH will lose a proton to become -COO- )
- The carboxylate ion (-COO-) will form a salt with a positively charged metal ion such as sodium (-COO-Na+)
- The clear blue colour of the solution turns opaque red due to the formation of a copper(I) oxide precipitate
- Ketones cannot be oxidised and therefore give a negative test when warmed with Fehling’s solution
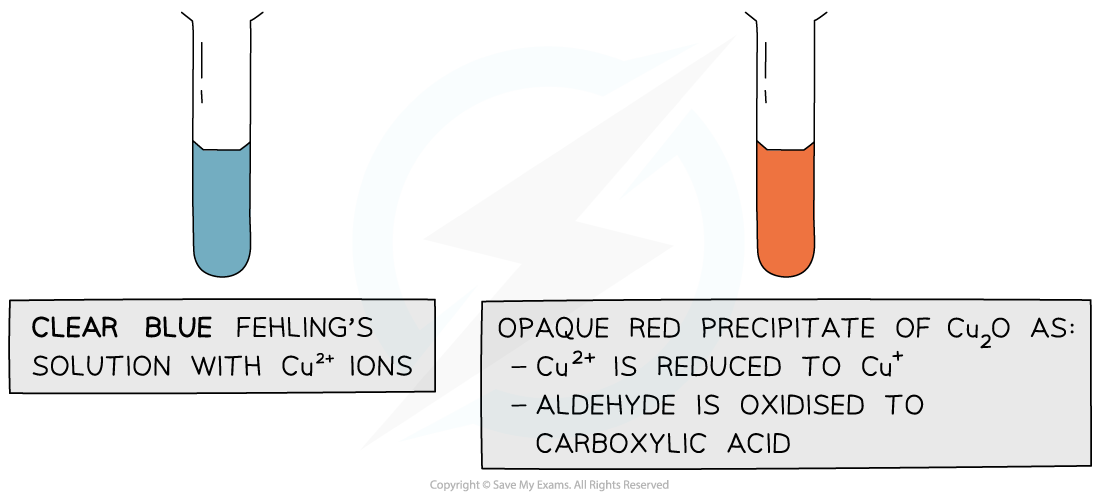
The copper(II) ions in Fehling’s solution are oxidising agents, oxidising the aldehyde to a carboxylic acid and getting reduced themselves to copper(I) ions in the Cu2O precipitate
Tollens’ reagent
- Tollens' reagent is an aqueous alkaline solution of silver nitrate in excess ammonia solution
- Tollen’s reagent is also called ammoniacal silver nitrate solution
- When warmed with an aldehyde, the aldehyde is oxidised to a carboxylic acid and the Ag+ ions are reduced to Ag atoms
- In the alkaline conditions, the carboxylic acid will become a carboxylate ion and form a salt
- The Ag atoms form a silver ‘mirror’ on the inside of the tube
- Ketones cannot be oxidised and therefore give a negative test when warmed with Tollens’ reagent
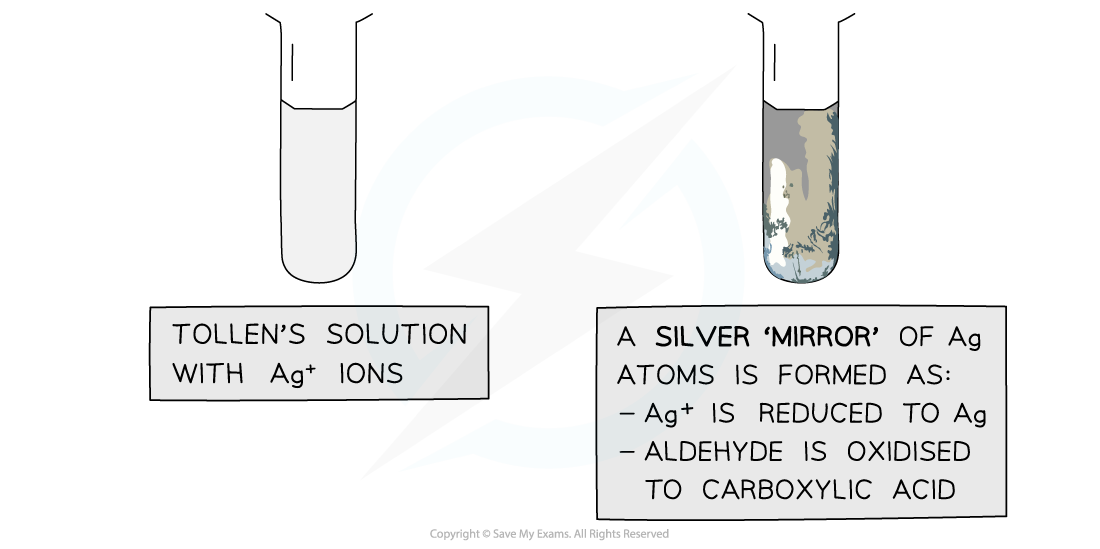
The Ag+ ions in Tollens’ reagent are oxidising agents, oxidising the aldehyde to a carboxylic acid and getting reduced themselves to silver atoms
转载自savemyexams

最新发布
© 2025. All Rights Reserved. 沪ICP备2023009024号-1









