- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
AQA A Level Chemistry复习笔记3.3.2 Reactions of Halogenoalkanes
The Key Reactions of the Halogenoalkanes
Types of Reactions that halogenoalkanes undergo:
- Halogenoalkanes are much more reactive than alkanes due to the presence of the electronegative halogens
- The carbon-halogen bond is polar causing the carbon to carry a partial positive and the halogen a partial negative charge
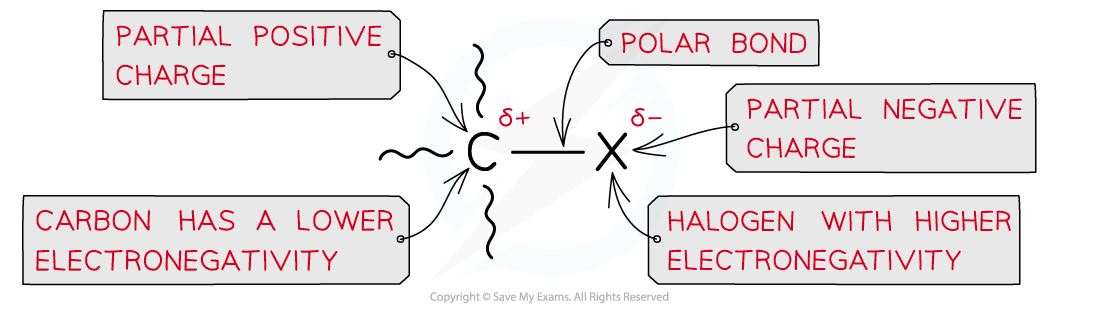
Due to the large difference in electronegativity between the carbon and halogen atom, the C-X bond is polar
- Because of this, halogenoalkanes will undergo two key types of reaction
- Nucleophilic substitution reactions
- A halogen is substituted for another atom or group of atoms
- The products formed when halogenoalkanes undergo this type of reaction are alcohols, amines and nitriles
- Elimination reactions
- A hydrogen halide is eliminated during the reaction
- The key product formed from this type of reaction is an alkene
Formation of alcohols
- The nucleophile in this reaction is the hydroxide, OH- ion
- An aqueous solution of sodium hydroxide (NaOH) or potassium hydroxide (KOH) with ethanol is used
- This reaction is very slow at room temperature, so the reaction mixture is warmed
- This is an example of a hydrolysis reaction and the product is an alcohol
- The rate of this reaction depends on the type of halogen in the halogenoalkane
- The stronger the C-X bond, the slower the rate of the reaction
- In terms of bond enthalpy, C-F > C-Cl > C-Br > C-I
- Fluoroalkanes do not react at all, but iodoalkanes have a very fast rate of reaction

The halogen is replaced by the nucleophile, OH-
- This reaction could also be done with water as the nucleophile, but it is very slow
- The hydroxide ion is a better nucleophile than water as it carries a full negative charge
- In water, the oxygen atom only carries a partial charge

A hydroxide ion is a better nucleophile as it has a full formal negative charge whereas the oxygen atom in water only carries a partial negative charge; this causes the nucleophilic substitution reaction with water to be much slower than the aqueous alkali
Measuring the rate of hydrolysis
- Acidified silver nitrate can be used to measure the rate of hydrolysis of halogenoalkanes
- Set up three test tubes in a 50 oC water bath, with a mixture of ethanol and acidified silver nitrate
- Add a few drops of a chloroalkane, bromoalkane and an iodoalkane to each test tube and start a stop watch
- Time how long it takes for the precipitates to form
- The precipitate will form as the reaction progresses and the halide ions are formed
- A white precipitate will form from the chloroalkane, a cream precipitate will form from the bromoalkane and a yellow precipitate will form from the iodoalkane
- The yellow precipitate will form the fastest
- This is because the C-I bond has the lowest bond enthalpy, so it is the easiest to break and will cause the I- ions to form the fastest
- The white precipitate will form the slowest
- This is because the C-Cl bond has the highest bond enthalpy, so it is the hardest to break and will cause the Cl- ions to form the slowest
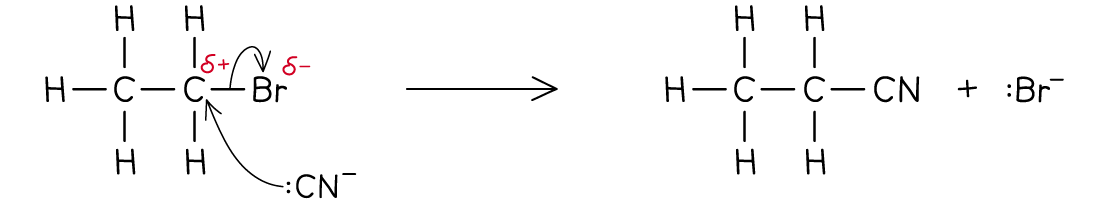
Formation of nitriles
- The nucleophile in this reaction is the cyanide, CN- ion
- Ethanolic solution of potassium cyanide (KCN in ethanol) is heated under reflux with the halogenoalkane
- The product is a nitrile
- Eg. bromoethane reacts with ethanolic potassium cyanide when heated under reflux to form propanenitrile

The halogen is replaced by a cyanide group, CN -
- The nucleophilic substitution of halogenoalkanes with KCN adds an extra carbon atom to the carbon chain
- This reaction can therefore be used by chemists to make a compound with one more carbon atom than the best available organic starting material
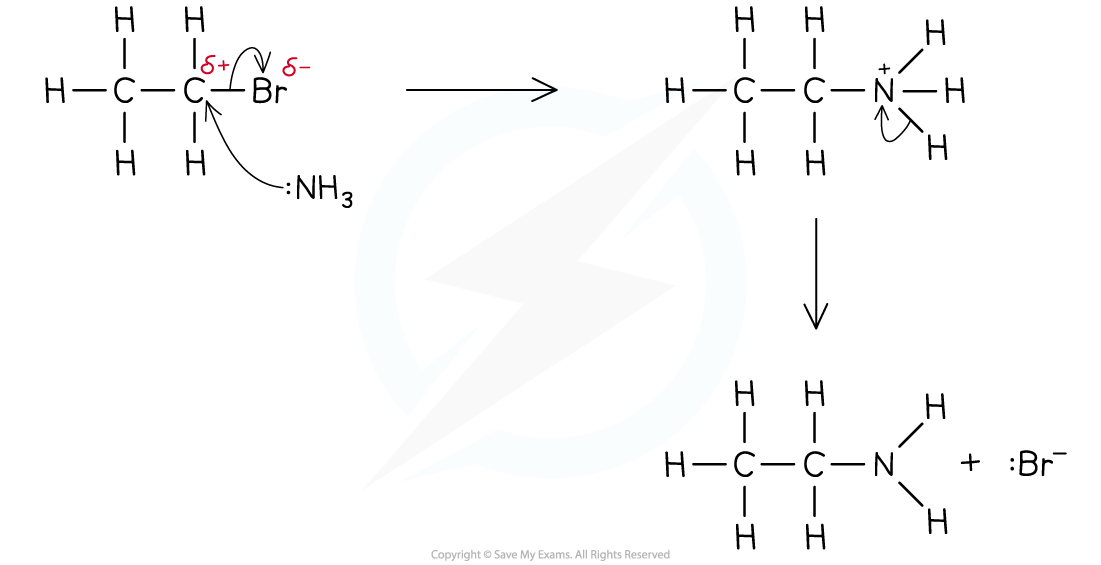
Formation of primary amines by reaction with ammonia
- The nucleophile in this reaction is the ammonia, NH3 molecule
- An ethanolic solution of excess ammonia (NH3 in ethanol) is heated under pressure with the halogenoalkane
- The product is a primary amine
- Eg. bromoethane reacts with excess ethanolic ammonia when heated under pressure to form ethylamine

The halogen is replaced by an amine group, NH2
Nucleophilic Substitution
- The nucleophilic substitution mechanisms for the above reactions are as follows:
Nucleophilic Substitution with OH-

Nucleophilic Substitution with NH3
Nucleophilic Substitution with CN-
Elimination
- In an elimination reaction, an organic molecule loses a small molecule
- In the case of halogenoalkanes this small molecule is a hydrogen halide (eg. HCl)
- The halogenoalkanes are heated with ethanolic sodium hydroxide causing the C-X bond to break heterolytically, forming an X- ion and leaving an alkene as an organic product
- For example, bromoethane reacts with ethanolic sodium hydroxide when heated to form ethene
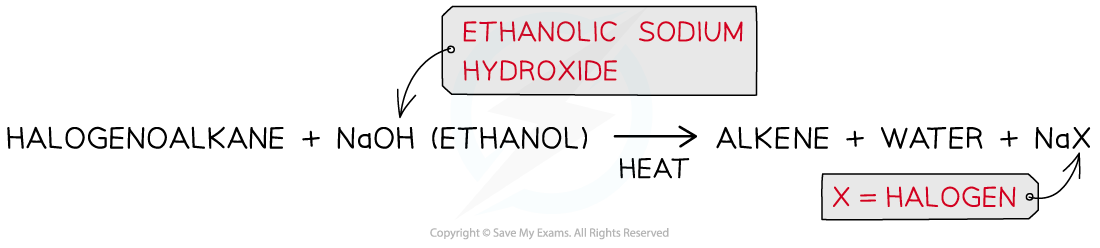
Production of an alkene from a halogenoalkane by reacting it with ethanolic sodium hydroxide and heating it
- For example, bromoethane reacts with ethanolic sodium hydroxide when heated to form ethene
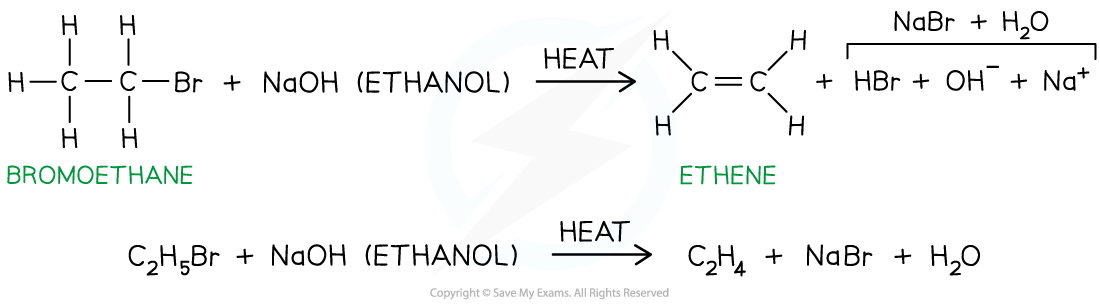
Hydrogen bromide is eliminated to form ethene
Elimination Reactions
- The elimination mechanism for the above reaction is as follows:
Elimination with OH-

Which product will form?
- Note that the reaction conditions should be stated correctly as different reaction conditions will result in different types of organic reactions
- NaOH (hot, in ethanol): an elimination reaction occurs to form an alkene
- NaOH (warm, aqueous): a nucleophilic substitution reaction occurs, and an alcohol is formed
转载自savemyexams

在线登记
最新发布
© 2025. All Rights Reserved. 沪ICP备2023009024号-1








