- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
AQA A Level Chemistry复习笔记3.1.5 Stereoisomerism
Stereoisomerism: Geometrical Isomerism
- Stereoisomers are compounds that have the same atoms connected to each other, however the atoms are differently arranged in space
- There are two types of stereoisomerism:
- Geometrical (E/Z) isomerism
- Optical isomerism (this will be covered in the Second Year of the A Level course)
Geometrical (E/Z) isomerism
- Geometrical isomerism is seen in unsaturated (double bond containing) or ring compounds that have the same molecular formula and order of atoms (the atoms are connected similarly to each other) but different shapes
- E/Z nomenclature is used to distinguish between the isomers
- Z isomers have functional groups on the same side of the double bond/carbon ring
- E isomers have functional groups on opposite sides of the double bond/carbon ring
- You may see this type of isomerism referred to in other sources as cis/trans isomerism
- This is a special case of E/Z isomerism
- A "cis" isomer would essentially be the same as a "Z" isomer and a "trans" isomer would also essentially be the same as an "E" isomer

Geometrical isomerism in unsaturated compounds
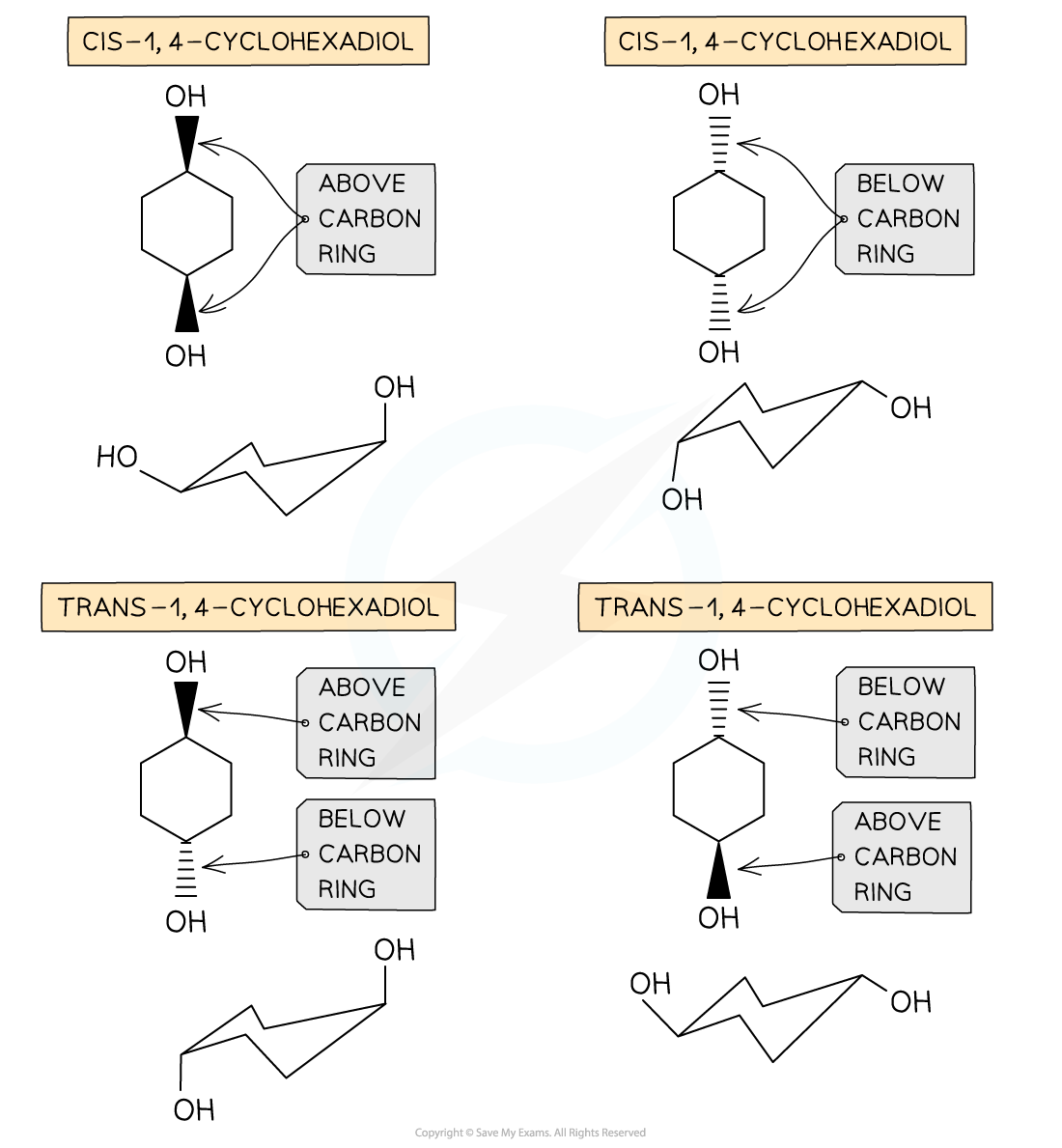
Geometrical isomerism in cyclic compounds
- This causes the compounds to have different chemical and physical properties
- For example, they may have different reaction rates for the same reaction (chemical property) or different melting/boiling points (physical property)
转载自savemyexams

早鸟钜惠!翰林2025暑期班课上线

最新发布
© 2025. All Rights Reserved. 沪ICP备2023009024号-1








