- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
AQA A Level Chemistry复习笔记2.2.3 Reactions of Group 2
Reactions of Group 2
- The group 2 elements react with oxygen, water and dilute acids
Group 2 Reactions - Observations
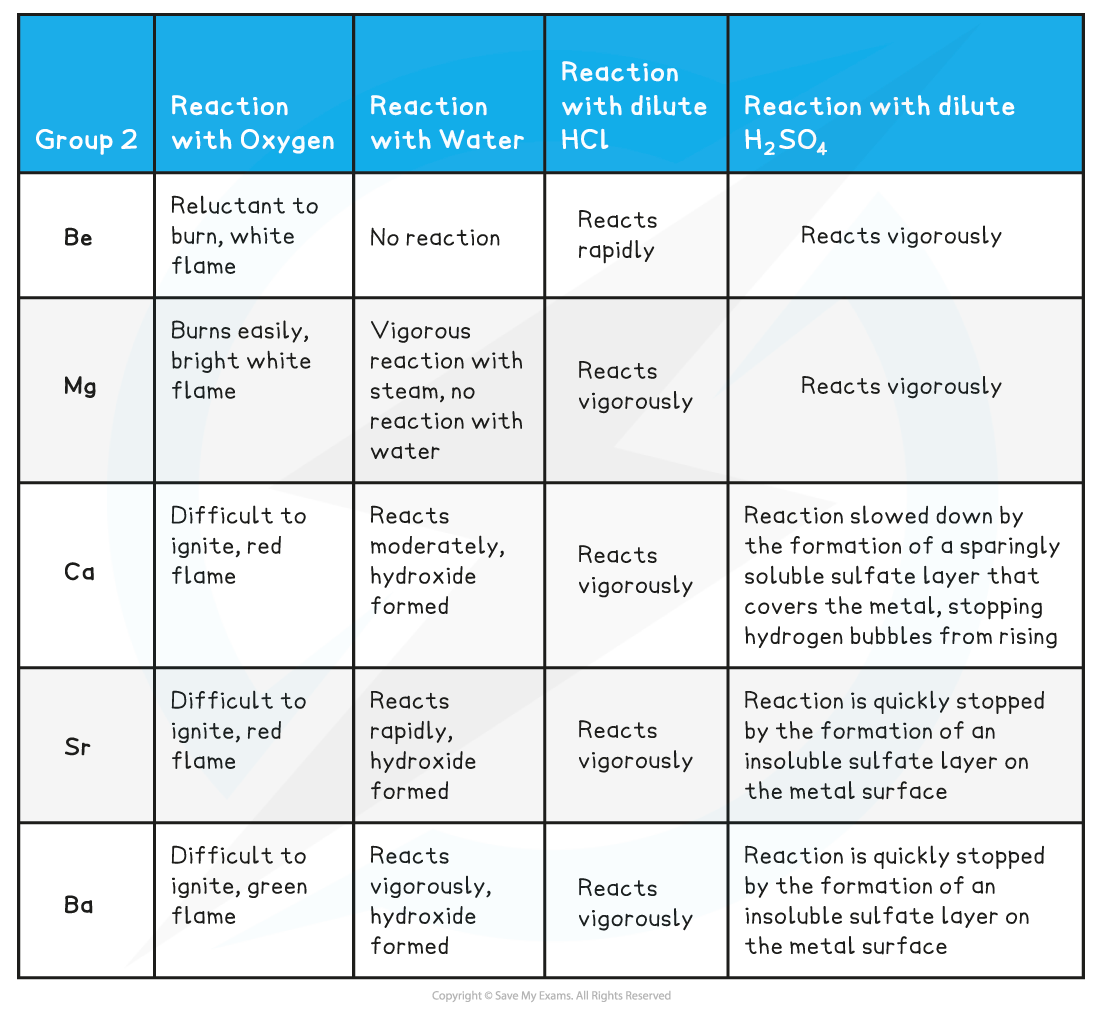
Reactions with water and oxygen
- The reaction of group 2 metals with oxygen follows the following general equation:
2M (s) + O2 (g) → 2MO (s)
Where M is any metal in group 2
Remember than Sr and Ba also form a peroxide, MO2
- The reaction of all metals with water follows the following general equation:
M (s) + 2H2O (l) → M(OH)2 (s) + H2 (g)
Except for, Be which does not react with water
Group 2 Metals reacting with Water and with Oxygen - Equations
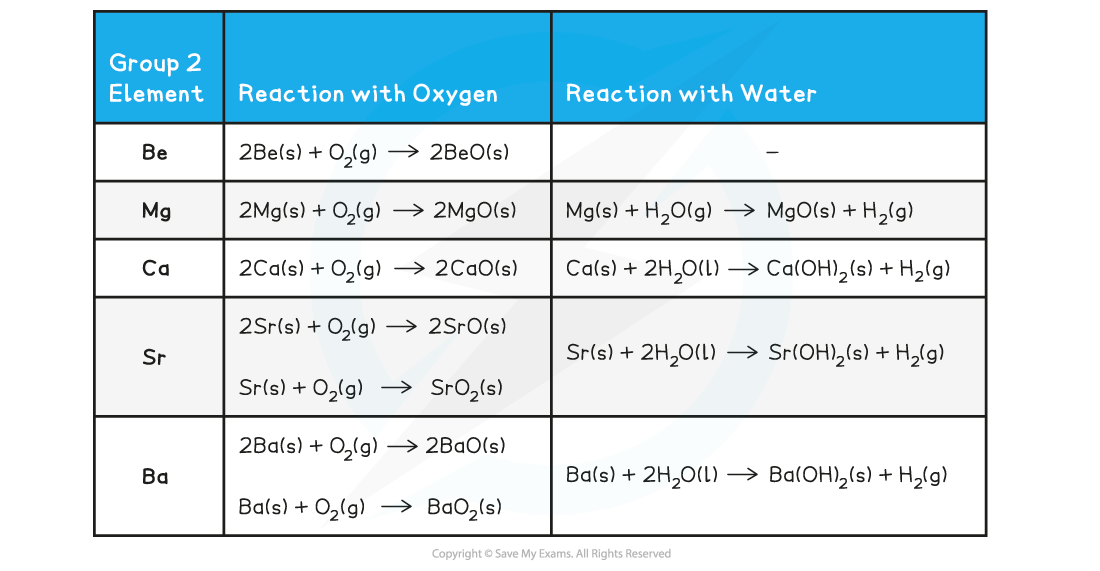
- Magnesium reacts extremely slowly with cold water:
Mg (s) + 2H2O (l) → Mg(OH)2 (aq) + H2 (g)
- The solution formed is weakly alkaline (pH 9-10) as magnesium hydroxide is only slightly soluble
- However, when magnesium is heated in steam, it reacts vigorously with steam to make magnesium oxide and hydrogen gas:
Mg (s) + H2O (g) → MgO (s) + H2 (g)
Reactions of Group 2 metals with acid
- The Group 2 metals will react with dilute acids to form colourless solutions of metal salts
- For example, they will form colourless solutions of metal chlorides if reacted with hydrochloric acid
- When metals react with an acid, the by-product of this reaction is hydrogen gas
Group 2 Reactions with Dilute Acids - Equations
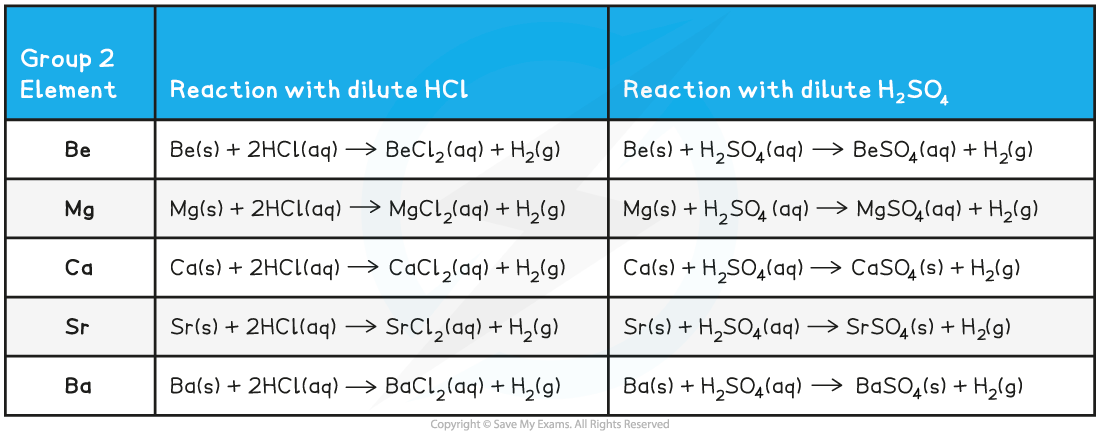
- When some of Group 2 metals react with sulfuric acid rather than hydrochloric, an insoluble sulfate forms
- Going down the group, the Group 2 sulfates become less and less soluble
- Calcium sulfate is sparingly soluble, but strontium sulfate and barium sulfate are insoluble
- The reaction of the metals with dilute HCl follows the following general equation:
M (s) + 2HCl (aq) → MCl2 (aq) + H2 (g)
- The reaction of the metals with dilute H2SO4 follows the following general equation:
M (s) + H2SO4 (aq) → MSO4 (aq) + H2 (g)
Remember that SrSO4 and BaSO4 are insoluble
Exam Tip
Learn the general equation for the reaction with oxygen, water and dilute HCl/H2SO4 and the exceptions instead of memorizing the entire table!
Group 2: Oxides, Hydroxides & Carbonates
Reactions of group 2 oxides with water
- All group 2 oxides are basic, except for BeO which is amphoteric (it can act both as an acid and base)
- Group 2 oxides react water to form alkaline solutions which get more alkaline going down the group
Group 2 Oxides reacting with Water
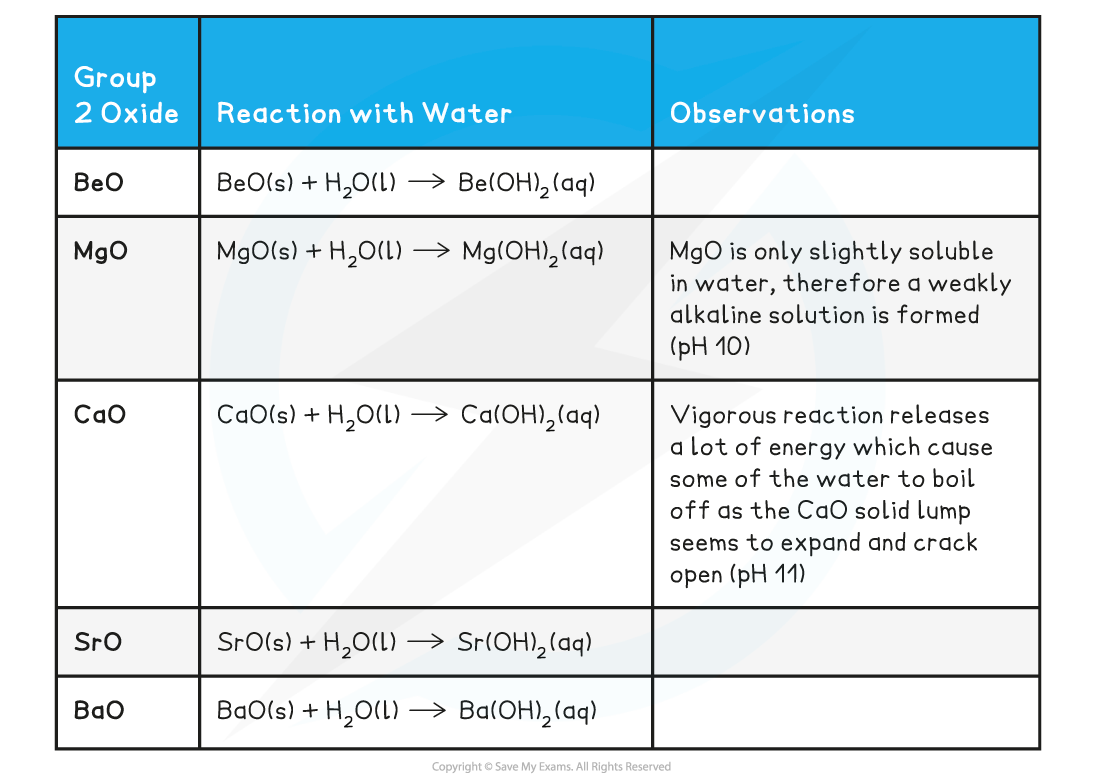
- Remember that:
oxide + water → hydroxide
- You should know that calcium hydroxide, when in solution, is also called limewater
Reactions of Group 2 oxides with acid
- Group 2 sulfates also form when a group 2 oxide is reacted with sulfuric acid
- The insoluble sulfates form at the surface of the oxide, which means that the solid oxide beneath it can’t react with the acid
- This can be prevented to an extent by using the oxide in powder form and stirring, in which case neutralisation can take place
- Remember that:
oxide + dilute hydrochloric acid → chloride + water
oxide + dilute sulfuric acid → sulfate + water
Reactions of group 2 hydroxides
- The group 2 metal hydroxides form colourless solutions of metal salts when they react with a dilute acid
- The sulfates decrease in solubility going down the group (barium sulfate is an insoluble white precipitate)
Group 2 Hydroxide Reactions with Dilute Acids
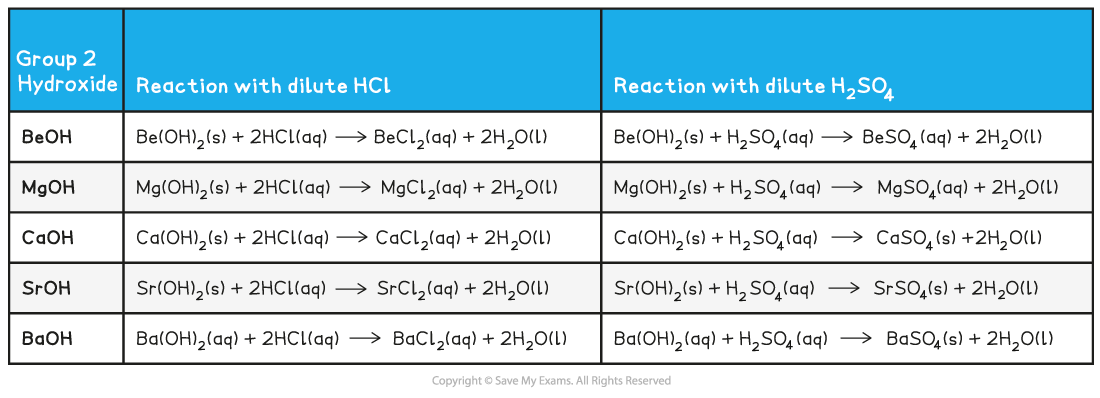
- Remember that:
hydroxide + dilute hydrochloric acid → chloride + water
hydroxide + dilute sulfuric acid → sulfate + water
Reactions of group 2 carbonates
- All group 2 carbonates (except for BeCO3) are insoluble in water
- All group 2 carbonates will form soluble chloride salts, water and carbon dioxide gas when reacted with dilute hydrochloric acid
- The carbonates of Ca, Sr and Ba form as an insoluble sulfate layer on their solid carbonates which stops any further reaction after the initial bubbling (effervescence) of carbon dioxide gas is seen
Group 2 Carbonates reacting with Dilute Acids
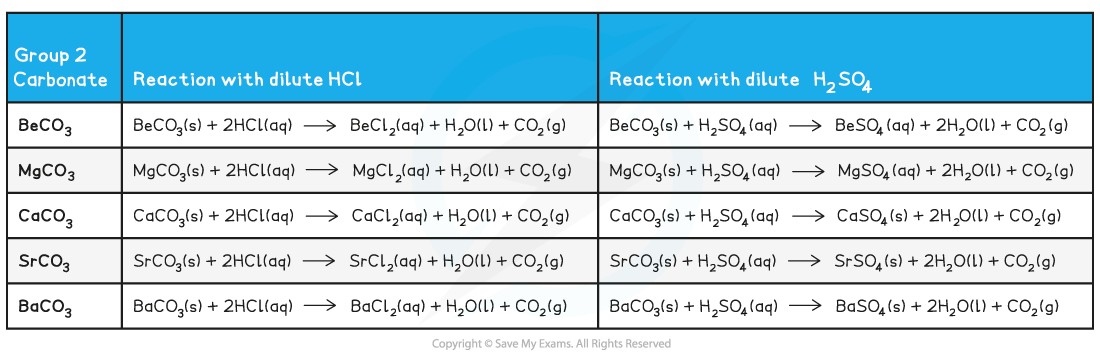
- Remember that:
carbonate + dilute hydrochloric acid → chloride + water + carbon dioxide
carbonate + dilute sulfuric acid → sulfate + water + carbon dioxide
转载自savemyexams

早鸟钜惠!翰林2025暑期班课上线

最新发布
© 2025. All Rights Reserved. 沪ICP备2023009024号-1








