- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
AQA A Level Chemistry复习笔记2.1.3 Trends of Period 3 Elements: First Ionisation Energy
Trend: First Ionisation Energy
- The first ionisation energy (IE1) is the energy required to remove one mole of electrons from one mole of atoms of an element in the gaseous state to form one mole of gaseous ions
- Eg. the first ionisation energy of Na is:
Na (g) → Na+ (g) + e-
IE1 Values of the Period 3 Elements
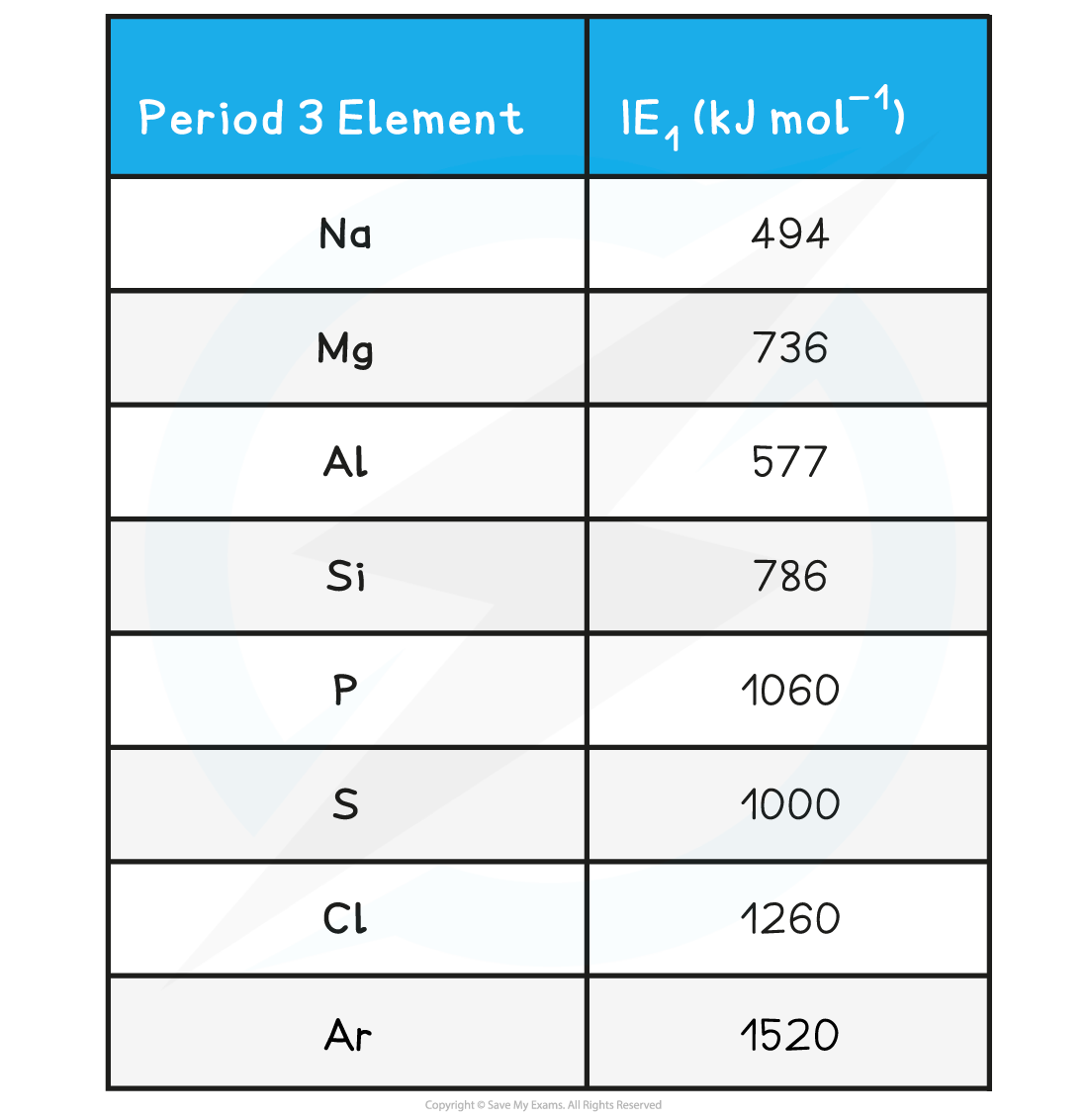
- There is a general increase in IE1across a period
- The nuclear charge increases
- The atomic radius decreases
- There are stronger attractive forces between the nucleus and outer electrons
- It therefore gets harder to remove any electrons
- Small ‘dips’ are observed between Mg - Al and P - S
转载自savemyexams

早鸟钜惠!翰林2025暑期班课上线

最新发布
© 2025. All Rights Reserved. 沪ICP备2023009024号-1








