- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
AQA A Level Chemistry复习笔记1.8.5 Changes Which Affect the Equilibrium
Changes Affecting the Equilibrium Constant
Changes in concentration
- If all other conditions stay the same, the equilibrium constant Kc is not affected by any changes in concentration of the reactants or products
- For example, the decomposition of hydrogen iodide:
2HI ⇌ H2 + I2
The equilibrium expression is:
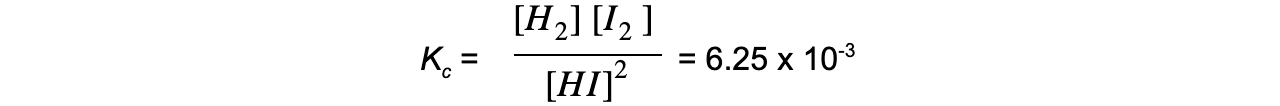
Adding more HI makes the ratio of [ products ] to [ reactants ] smaller
To restore equilibrium, [H2] and [I2] increases and [HI] decreases
Equilibrium is restored when the ratio is 6.25 x 10-3 again
Changes in pressure
- A change in pressure only changes the position of the equilibrium (see Le Chatelier’s principle)
- If all other conditions stay the same, the equilibrium constant Kc is not affected by any changes in pressure of the reactants and products
Changes in temperature
- Changes in temperature affect the value of the equilibrium constant, Kc
- For an endothermic reaction such as:
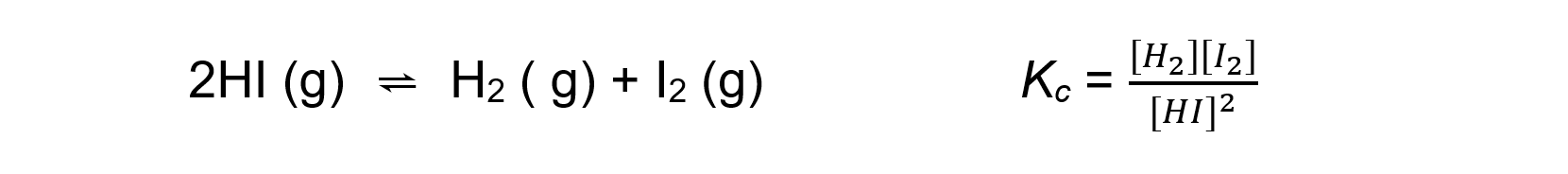
An increase in temperature:
[H2] and [I2] increases
[HI] decreases
Because [H2] and [I2] are increasing and [HI] is decreasing, the equilibrium constant Kc increases
- For an exothermic reaction such as:
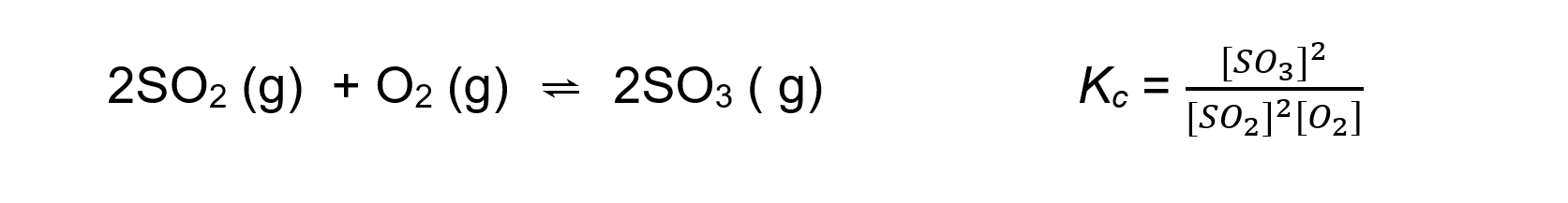
An increase in temperature:
[SO3] decreases
[SO2] and [O2] increases
Because [SO3] decreases and [SO2] and [O2] increases the equilibrium constant Kc decreases
Presence of a catalyst
- If all other conditions stay the same, the equilibrium constant Kc is not affected by the presence of a catalyst
- A catalyst speeds up both the forward and reverse reactions at the same rate so the ratio of [ products ] to [ reactants ] remains unchanged
Worked Example
Factors affecting Kc
An equilibrium is established in the reaction
AB (aq) + CD (aq) ⇌ AC (aq) + BD (aq) ΔH = +180 kJ mol-1
Which factors would affect the value of Kc in this equilibrium?
Answer
-
- Only a change in temperature will affect the value of Kc and any other changes in conditions would result in the position of the equilibrium moving in such way to oppose this change.
- Adding a catalyst will increase the rate of reaction meaning the state of equilibrium will be reached faster but will have no effect on the position of the equilibrium and therefore Kc is unchanged.
Choosing Conditions to Maximise Yield
- Equilibrium reactions are involved in some stages of the large-scale production of certain chemicals
- An understanding of equilibrium and Le Chatelier’s principle is therefore very important in the chemical industry
Haber process
- The Haber process involves the synthesis of ammonia according to:
N2 (g) + 3H2 (g) ⇌ 2NH3 (g) ΔHr = -92 kJ mol-1
- Le Chatelier’s principle is used to get the best yield of ammonia
Maximising the ammonia yield
Pressure
- An increase in pressure will result in the equilibrium shifting in the direction of the fewest molecules of gas formed to reduce the pressure
- In this case, the equilibrium shifts towards the right so the yield of ammonia increases
- An increase in pressure will cause the particles to be closer together and therefore increasing the number of successful collisions leading to an increased reaction rate
- Very high pressures are expensive to produce therefore a compromise pressure of 200 atm is chosen
Temperature
- To get the maximum yield of ammonia the position of equilibrium should be shifted as far as possible to the right as possible
- Since the Haber process is an exothermic reaction, according to Le Chatelier’s principle the equilibrium will shift to the right if the temperature is lowered
- A decrease in temperature will decrease the energy of the surroundings so the reaction will go in the direction in which energy is released to counteract this
- Since the reaction is exothermic, the equilibrium shifts to the right
- However, at a low temperature the gases won’t have enough kinetic energy to collide and react and therefore equilibrium would not be reached therefore a compromise temperature of 400-450oC is used in the Haber process
- A heat exchanger warms the incoming gas mixture to give molecules more kinetic energy such that the gas molecules collide more frequently increasing the likelihood of a reaction
Removing ammonia
- Removing ammonia by condensing it to a liquid causes the equilibrium position to shift to the right to replace the ammonia causing more ammonia to be formed from hydrogen and nitrogen
- The recovered ammonia is stored at very low temperatures and there is no catalyst present with the stored ammonia so the decomposition reaction of ammonia to decompose back into hydrogen and nitrogen will be too slow to be a problem
Catalysts
- In the absence of a catalyst the reaction is so slow that hardly anything happens in a reasonable time!
- Adding an iron catalyst speeds up the rate of reaction
Contact process
- The Contact process involves the synthesis of sulfuric acid according to:
2SO2(g) + O2(g) ⇌ 2SO3(g) ΔHr = -197 kJ mol-1
SO3 + H2SO4 → H2S2O7
H2S2O7 + H2O → 2H2SO4
- Le Chatelier’s principle is used to get the best yield of sulfuric acid
Maximising the sulfuric acid yield
Pressure
- An increase in pressure will result in the equilibrium shifting in the direction of the fewest molecules of gas formed to reduce the pressure
- In this case, the equilibrium shifts towards the right so the yield of sulfur trioxide increases
- In practice, the reaction is carried out at only 1 atm
- This is because Kc for this reaction is already very high meaning that the position of the equilibrium is already far over to the right
- Higher pressures than 1 atm will be unnecessary and expensive
Temperature
- The same principle applies to increasing the temperature in the Contact process as in the Haber process
- A compromise temperature of 450 oC is used
Removing sulfuric acid
- In practice, SO3 is removed by absorbing it in 98% sulfuric acid
- This is because adding SO3 directly to water produces an uncontrollable and hazardous exothermic reaction
- The SO3 reacts with the sulfuric acid to make oleum, H2S2O7, which is then carefully diluted to make more H2SO4
Catalysts
- The Contact process uses vanadium(V) oxide as a catalyst to increase the rate of reaction
转载自savemyexams

最新发布
© 2026. All Rights Reserved. 沪ICP备2023009024号-1









