- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
AQA A Level Chemistry复习笔记1.7.5 Effect of Concentration & Pressure
Concentration, Pressure & Rate of Reaction
Concentration
- The more concentrated a solution is, the greater the number of particles in a given volume of solvent
- An increase in concentration causes in an increased collision frequency and therefore an increased rate of reaction
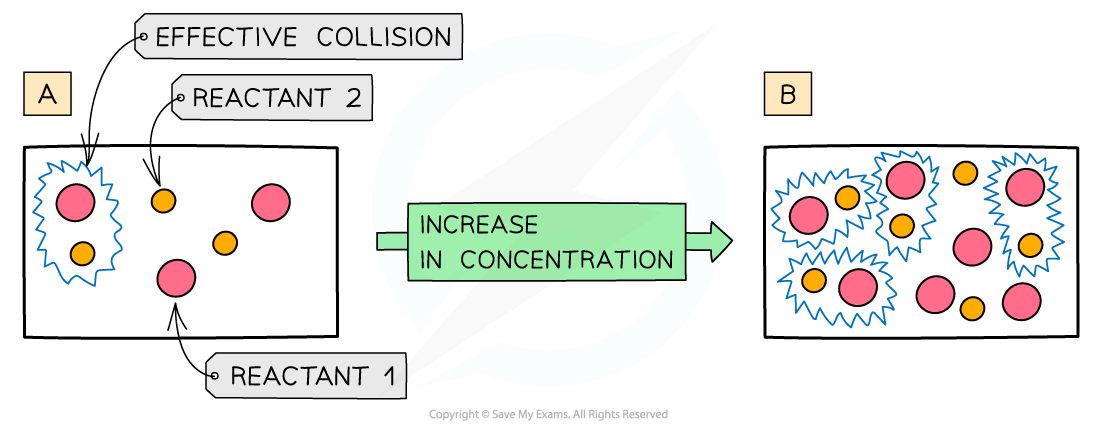
The diagram shows a higher concentration of particles in (b) which means that there are more particles present in the same volume than (a) so the chances and frequency of collisions between reacting particles is increased causing an increased rate of reaction
Pressure
- An increase in pressure in reactions that involve gases has the same effect as an increase in the concentrations of solutions
- When the pressure is increased, the molecules have less space in which they can move
- This means that the number of effective collisions increases due to an increased collision frequency
- An increase in pressure therefore increases the rate of reaction
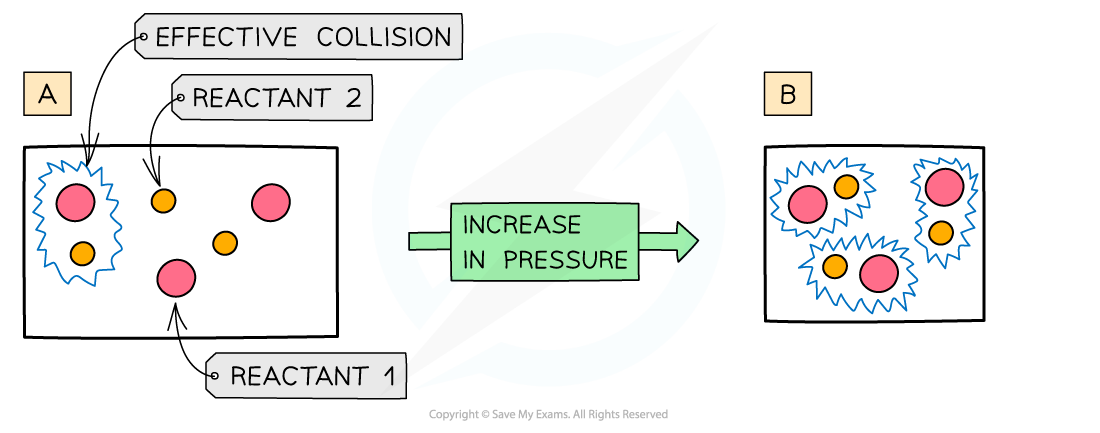
The diagram shows a higher pressure in (b) which means that the same number of particles occupy a smaller volume, resulting in an increased collision frequency and therefore increased rate of reaction
转载自savemyexams

早鸟钜惠!翰林2025暑期班课上线

最新发布
© 2025. All Rights Reserved. 沪ICP备2023009024号-1








