- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
AQA A Level Chemistry复习笔记1.7.3 Maxwell–Boltzmann Distributions
Maxwell-Boltzmann Distribution Curve - Temperature
Maxwell-Boltzmann distribution curve
- A Maxwell-Boltzmann distribution curve is a graph that shows the distribution of energies at a certain temperature
- In a sample of a gas, a few particles will have very low energy, a few particles will have very high energy, but most particles will have energy in between
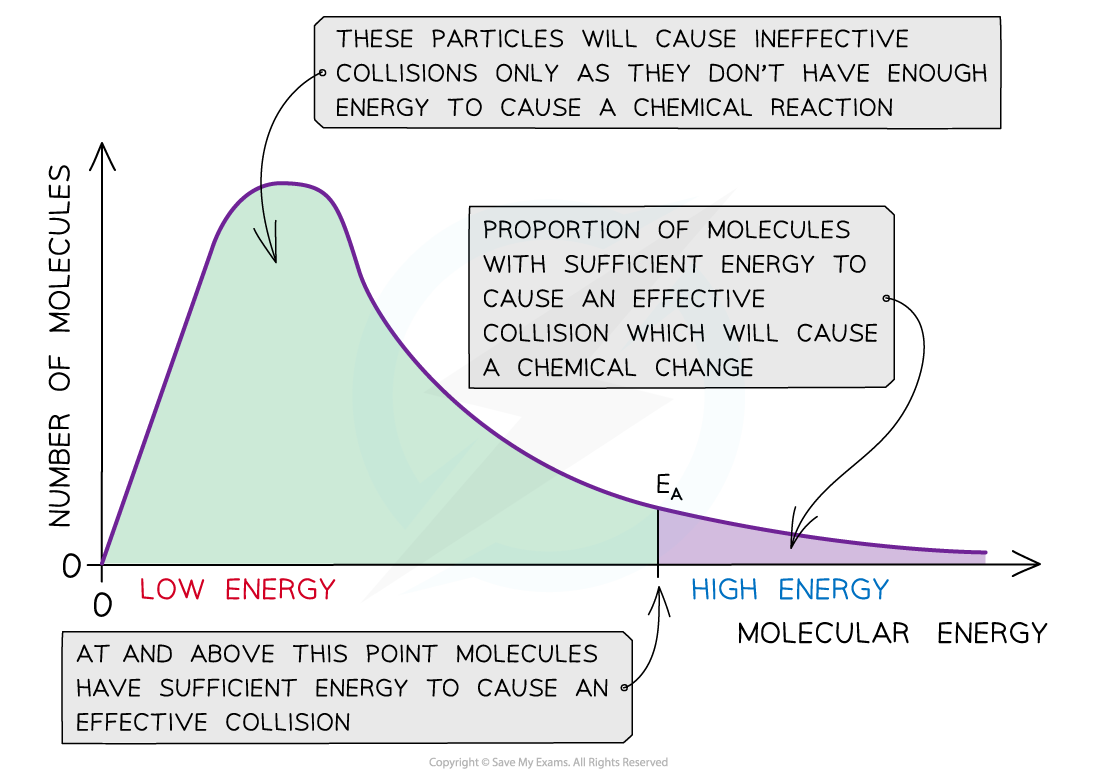
The Maxwell-Boltzmann distribution curve shows the distribution of the energies and the activation energy
- The graph shows that only a small proportion of molecules in the sample have enough energy for an effective collision and for a chemical reaction to take place
Changes in temperature
- When the temperature of a reaction mixture is increased, the particles gain more kinetic energy
- This causes the particles to move around faster resulting in more frequent collisions
- Furthermore, the proportion of successful collisions increases, meaning a higher proportion of the particles possess the minimum amount of energy (activation energy) to cause a chemical reaction
- With higher temperatures, the Boltzmann distribution curve flattens and the peak shifts to the right
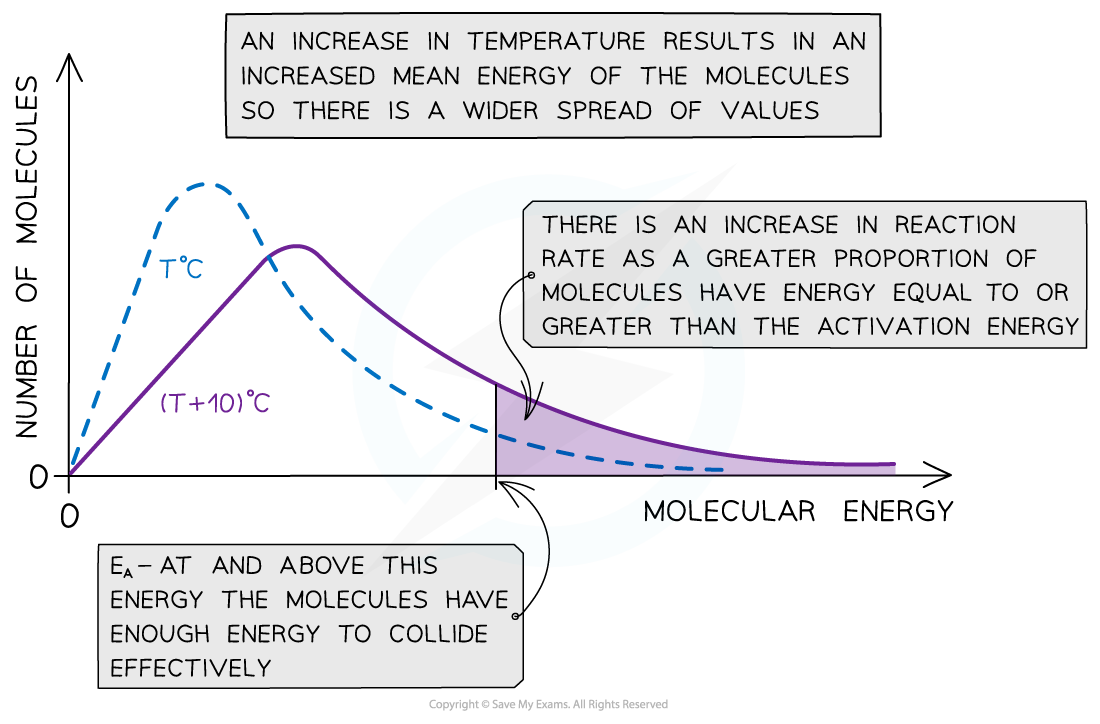
The Maxwell-Boltzmann distribution curve at T oC and when the temperature is increased by 10 oC
- Therefore, an increase in temperature causes an increased rate of reaction due to:
- There being more effective collisions as the particles have more kinetic energy, making them move around faster
- A greater proportion of the molecules having kinetic energy greater than the activation energy
Exam Tip
The increase in proportion of molecules having kinetic energy greater than the activation has a greater effect on the rate of reaction than the increase in effective collisions
转载自savemyexams

最新发布
© 2026. All Rights Reserved. 沪ICP备2023009024号-1









