- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
AQA A Level Chemistry复习笔记1.6.6 Applications of Hess’s Law
Hess' Law Calculations
- You must make sure that you can apply Hess' Law effectively and calculate enthalpy changes in different situations
- Remember - it is the data that is important
- Check whether the data you have been given is formation data or combustion data, and then complete the cycle or calculation according to that
Worked Example
Calculating the enthalpy change of formation of ethaneCalculate ΔHf [ethane]. The relevant change in standard enthalpy of combustion (ΔHc) values are shown in the table below: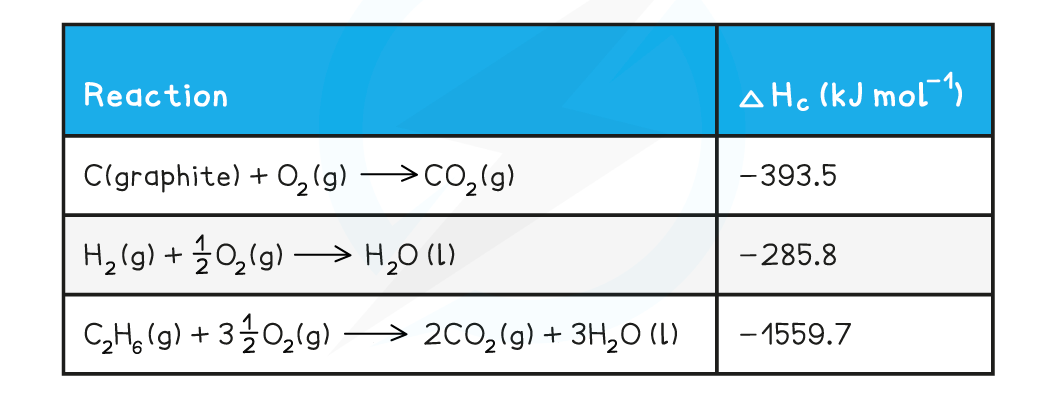
Answer
Step 1: Write the equation for enthalpy change of formation at the top and add oxygen on both sides
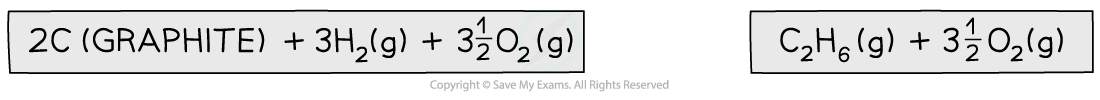
Step 2: Draw the cycle with the combustion products at the bottom
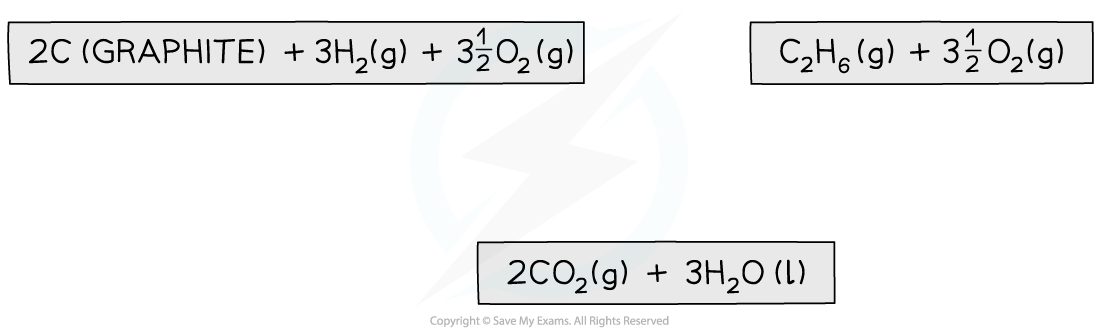
Step 3: Draw all arrows in the correct direction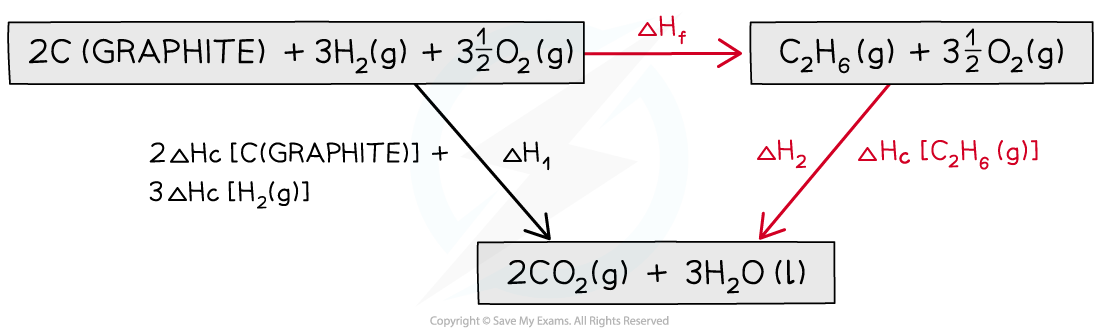
Step 4: Apply Hess’s Law

Calculating average bond energies using Hess’s cycles
- Bond energies cannot be found directly so enthalpy cycles are used to find the average bond energy
- This can be done using enthalpy changes of atomisation and combustion or formation
- The enthalpy change of atomisation (ΔHatꝋ ) is the enthalpy change when one mole of gaseous atoms is formed from its elements under standard conditions.
- Eg. ΔHatꝋ [H2] relates to the equation:
½ H2(g) → H(g)
Worked Example
Calculating average C-H bond energyCalculate the average bond energy of the C-H bond using the relevant ΔHfꝋ and ΔHatꝋ values in the table below: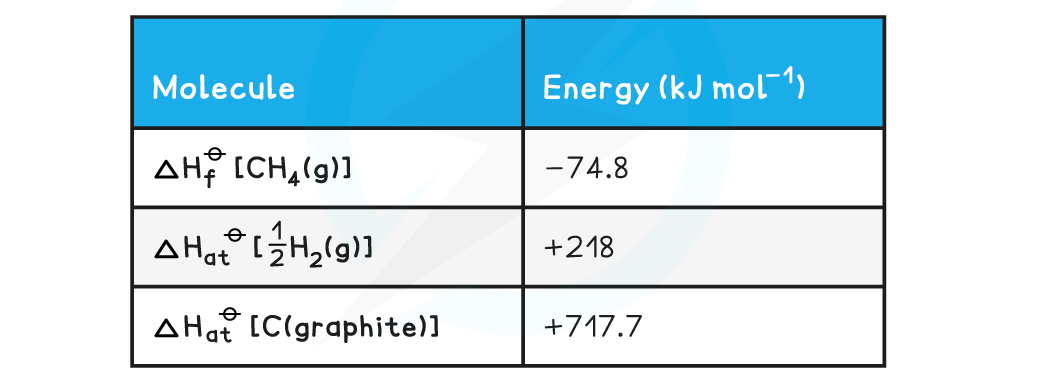
Answer
Step 1: Write down the equation for the dissociation of methane at the top

Step 2: Write down the elements at the bottom
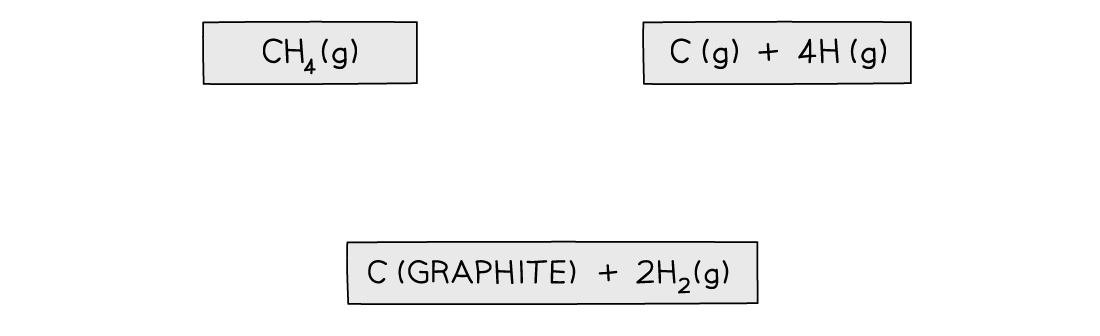
Step 3: Draw all arrows in the correct direction
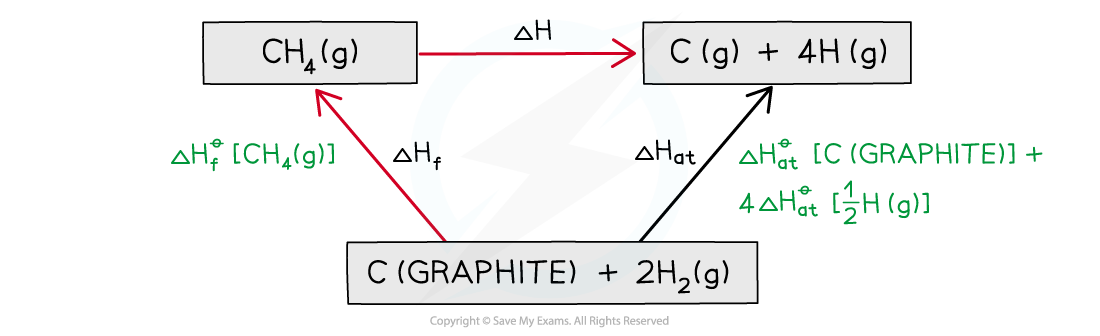
Step 4: Apply Hess’s Law
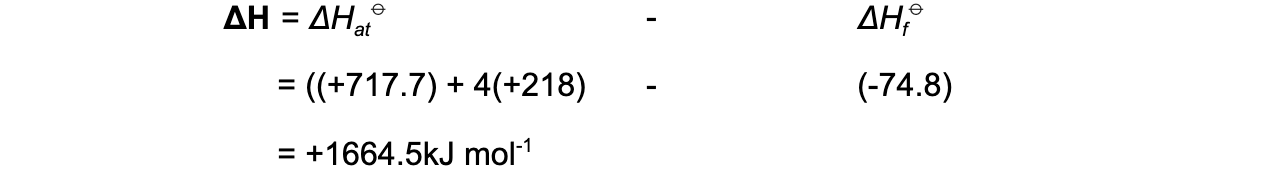
Step 5: Since there are 4 C-H bonds in methane:
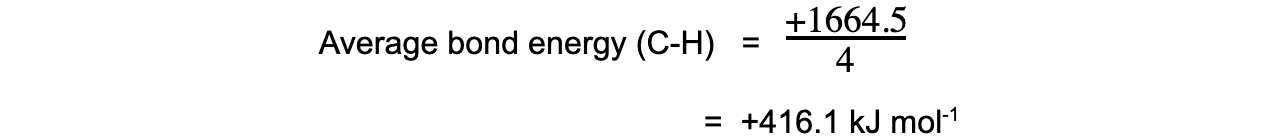
Exam Tip
Remember to take into account the number of moles of each reactant and product.For example, there are two moles of NaHCO3(s) so the ΔHf value is multiplied by 2.
转载自savemyexams

最新发布
© 2026. All Rights Reserved. 沪ICP备2023009024号-1









