- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
AQA A Level Chemistry复习笔记1.6.5 Hess' Law
Using Hess' Law
Calculating ΔHr from ΔHf using Hess’s Law energy cycles
- The products can be directly formed from the elements = ΔH2
OR
- The products can be indirectly formed from the elements = ΔH1 + ΔHr
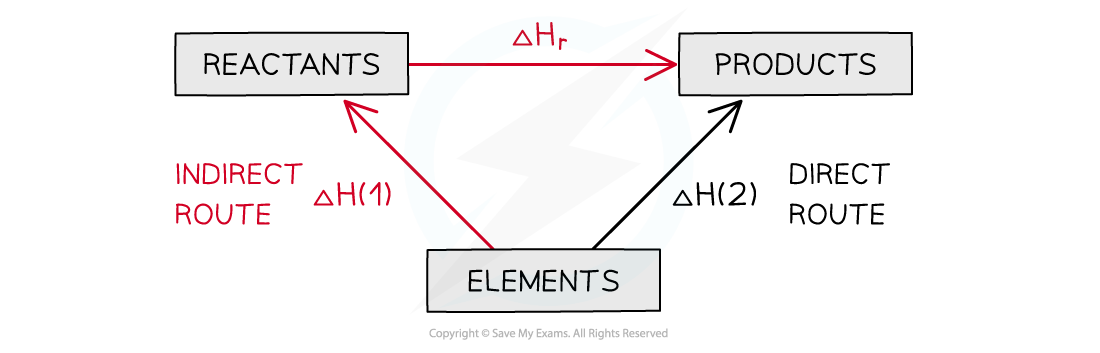
The enthalpy change from elements to products (direct route) is equal to the enthalpy change of elements forming reactants and then products (indirect route)
- Equation
ΔH2 = ΔH1 + ΔHr
Therefore,
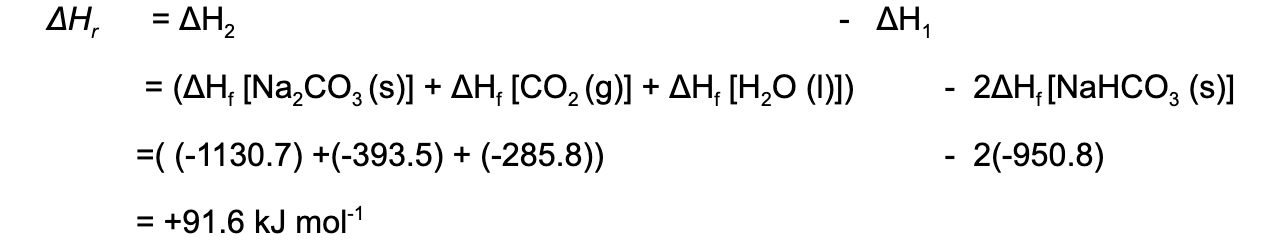
ΔHr = ΔH2 – ΔH1
Worked Example
Calculating the enthalpy change of reaction
Calculate the ΔHf for the following reaction:
2NaHCO3 (s) → Na2CO3 (s) + CO2 (g) + O(I)
The table below shows the standard enthalpy of formations (ΔHfꝋ) relevant to this reaction: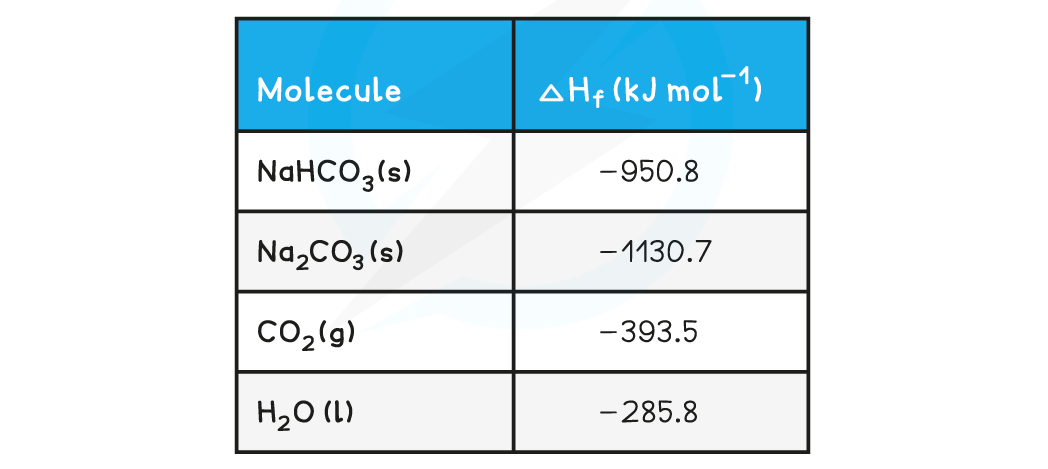
Answer
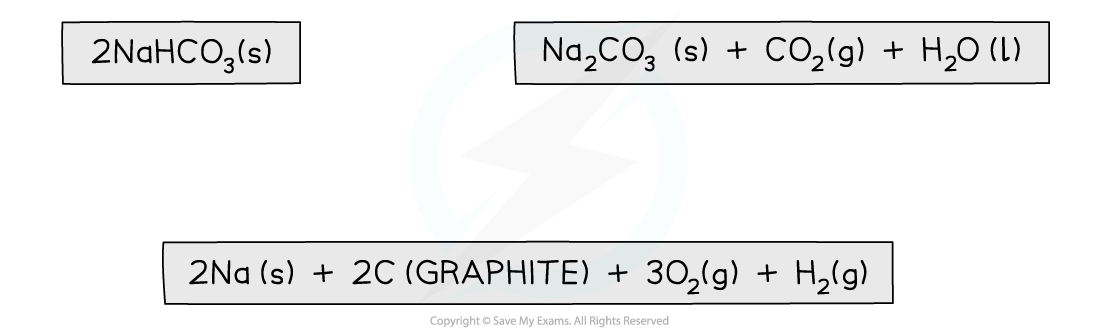
Step 1: Write the balanced equation at the top

Step 2: Draw the cycle with the elements at the bottom
Step 3: Draw in all arrows, making sure they go in the correct directions. Write the standard enthalpy of formations
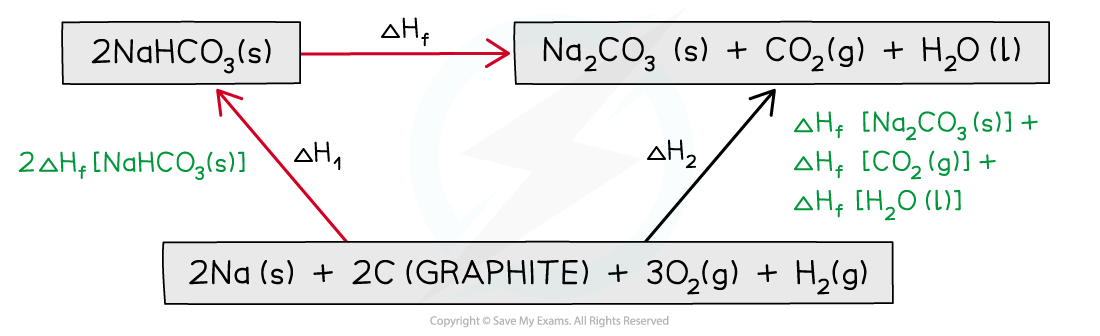
Step 4: Apply Hess’s Law
Calculating average bond energies using Hess's cycles
- Bond energies cannot be found directly so enthalpy cycles are used to find the average bond energy
- This can be done using enthalpy changes of atomisation and combustion or formation
- The enthalpy change of atomisation (ΔHatꝋ ) is the enthalpy change when one mole of gaseous atoms is formed from its elements under standard conditions.
- Eg. ΔHatꝋ [H2] relates to the equation:
½ H2(g) → H(g)
转载自savemyexams

最新发布
© 2026. All Rights Reserved. 沪ICP备2023009024号-1









