- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
AQA A Level Chemistry复习笔记1.6.3 Enthalpy Changes
Enthalpy Changes
- The total chemical energy inside a substance is called the enthalpy (or heat content)
- When chemical reactions take place, changes in chemical energy take place and therefore the enthalpy changes
- An enthalpy change is represented by the symbol ΔH (Δ= change; H = enthalpy)
- An enthalpy change can be positive or negative
Exothermic reactions
- A reaction is exothermic when the products have less energy than the reactants
- Heat energy is given off by the reaction to the surroundings
- The temperature of the environment increases - this can be measured with a thermometer
- The energy of the system decreases
- There is an enthalpy decrease during the reaction so ΔH is negative
- Exothermic reactions are thermodynamically possible (because the enthalpy of the reactants is higher than that of the products)
- However, if the rate is too slow, the reaction may not occur
- In this case the reaction is kinetically controlled
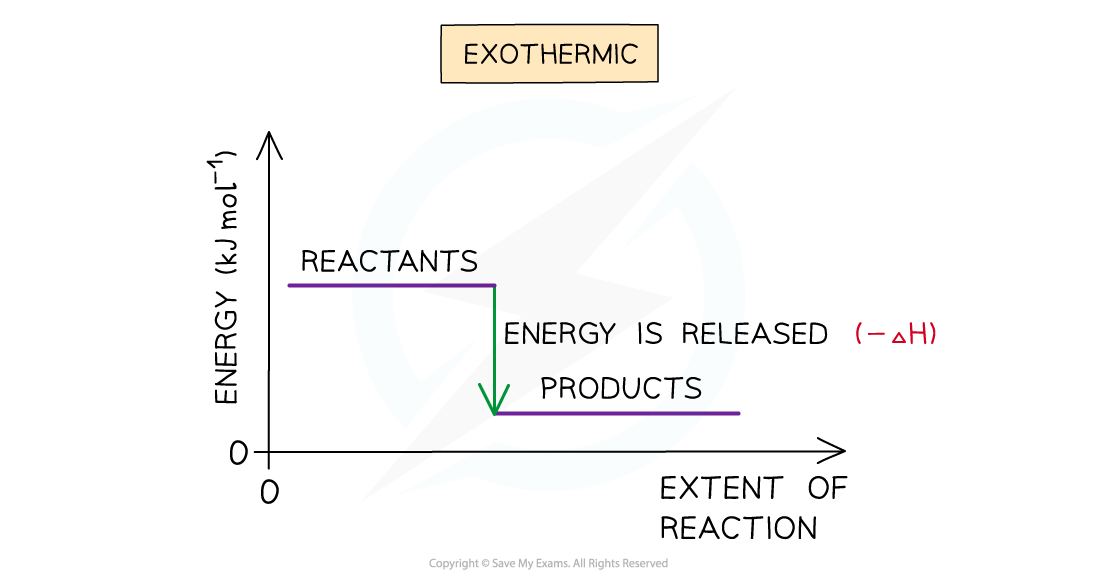
The enthalpy change during an exothermic reaction
Endothermic reactions
- A reaction is endothermic when the products have more energy than the reactants
- Heat energy is absorbed by the reaction from the surroundings
- The temperature of the environment decreases - this can be measured with a thermometer
- The energy of the system increases
- There is an enthalpy increase during the reaction so ΔH is positive
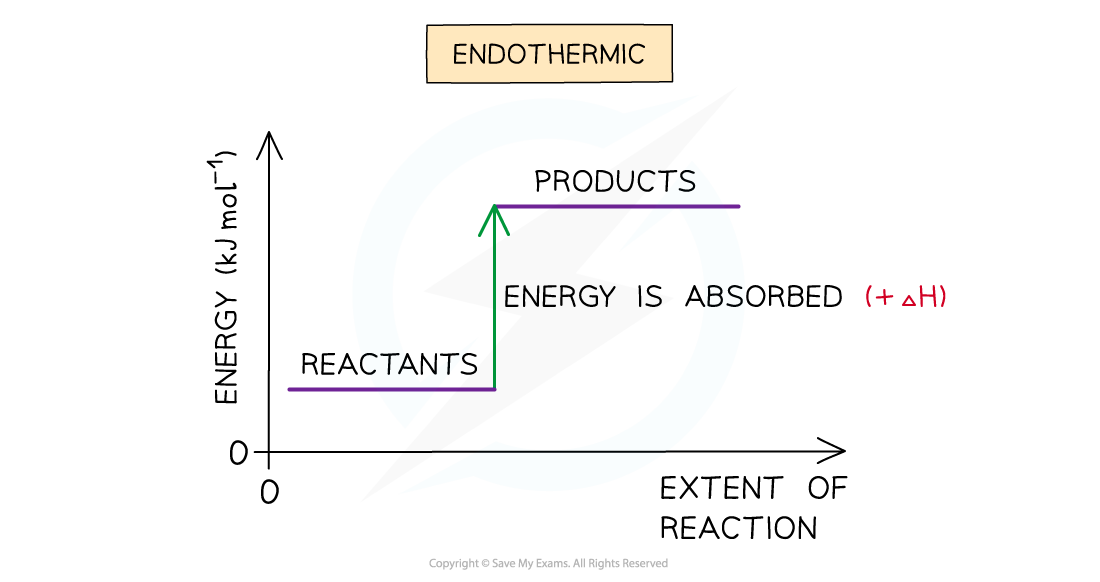
The enthalpy change during an endothermic reaction
Exam Tip
It is important to specify the physical states of each species in an equation when dealing with enthalpy changes as any changes in state can cause very large changes of enthalpy. For example:
NaCl (s) → Na+ (aq) + Cl- (aq) ΔH = +4 kJ mol-1
NaCl (g) → Na+ (g) + Cl- (g) ΔH = +500 kJ mol-1
Also, remember that the system is the substances that are reacting (i.e. the reaction itself) and the surroundings is everything else (e.g. the flask the reaction is taking place in).
Standard Enthalpy Changes
- To be able to compare the changes in enthalpy between reactions, all thermodynamic measurements are carried out under standard conditions
- These standard conditions are:
- A pressure of 100 kPa (you may see some older exam questions that use a figure of 101 kPa; the exact figure is 101 325 Pa, but it has been simplified in the current syllabus for problem-solving purposes)
- A temperature of 298 K (25 oC)
- Each substance involved in the reaction is in its standard physical state (solid, liquid or gas)
- To show that a reaction has been carried out under standard conditions, the symbol Ꝋ is used
- ΔHꝊ = the standard enthalpy change
- There are a number of key definitions relating to enthalpy changes that you need to know
Enthalpy Definitions Table
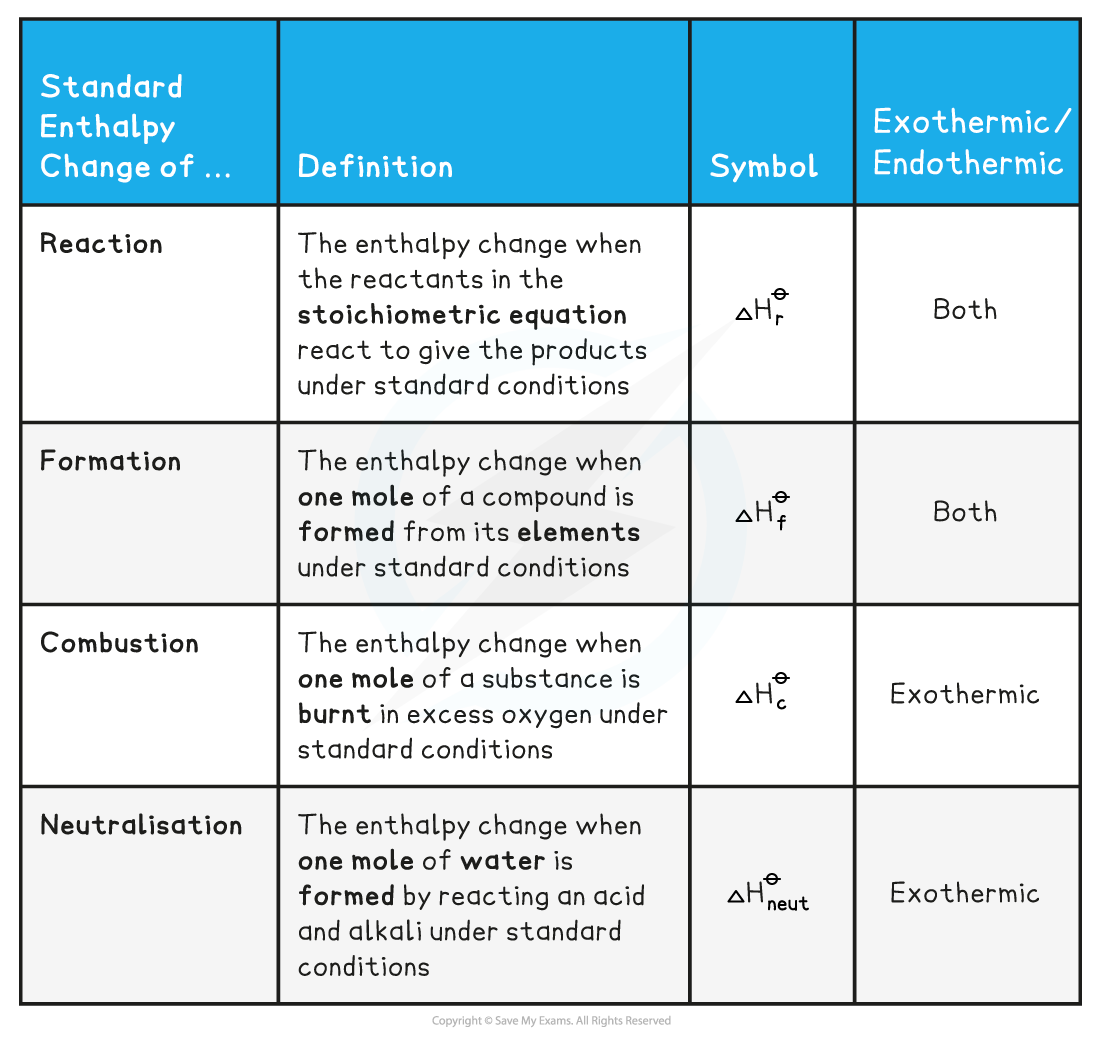
Worked Example
Calculating the enthalpy change of reaction
One mole water is formed from hydrogen and oxygen, releasing 286 kJ of energy
H2 (g) + ½O2 (g) → H2O (I) ΔHrꝊ = -286 kJ mol-1
Calculate ΔHrꝊ for the reaction below:
2H2 (g) + O2 (g) → 2H2O (I)
Answer
-
- Since two moles of water molecules are formed in the question above, the energy released is simply:
-
- ΔHrꝊ = 2 mol x (-286 kJ mol-1) = -572 kJ mol-1
Worked Example
Calculating the enthalpy change
Calculate ΔHfꝋ for the reaction below, given that ΔHfꝋ [Fe2O3(s)] = -824.2 kJ mol-1
4Fe(s) + 3 O2(g) → 2 Fe2O3(s)
Answer
-
- Since two moles of Fe2O3 (s) are formed the total change in enthalpy for the reaction above is:
- ΔHfꝊ = 2 x ( -824.2 kJ mol-1) = - 1648 kJ
Worked Example
Calculating enthalpy changes
Identify each of the following as ΔHrꝊ, ΔHfꝊ, ΔHcꝊ or ΔHneutꝊ
- MgCO3 (s) → MgO (s) + CO2 (g)
- C (graphite) + O2 (g) → CO2 (g)
- HCl (aq) + NaOH (aq) → NaCl (aq) + H2O (I)
Answer
Answer 1: ΔHrꝊ
Answer 2: ΔHfꝊ as one mole of CO2 is formed from its elements in standard state and ΔHcꝊ as one mole of carbon is burnt in oxygen
Answer 3: ΔHneutꝊ as one mole of water is formed from the reaction between an acid and an alkali
Exam Tip
The ΔHfꝊ of an element in its standard state is zero.
For example, ΔHfꝊ of O2(g) is 0 kJ mol-1
转载自savemyexams

最新发布
© 2025. All Rights Reserved. 沪ICP备2023009024号-1









