- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
AQA A Level Chemistry复习笔记1.4.4 Dative Covalent Bonding
Dative Covalent Bonding
- In simple covalent bonds, the two atoms involved share electrons
- Some molecules have a lone pair of electrons that can be donated to form a bond with an electron-deficient atom
- An electron-deficient atom is an atom that has an unfilled outer orbital
- So both electrons are from the same atom
- This type of bonding is called dative covalent bonding or coordinate bonding
- An example with a dative bond is in an ammonium ion
- The hydrogen ion, H+ is electron-deficient and has space for two electrons in its shell
- The nitrogen atom in ammonia has a lone pair of electrons which it can donate to the hydrogen ion to form a dative covalent bond
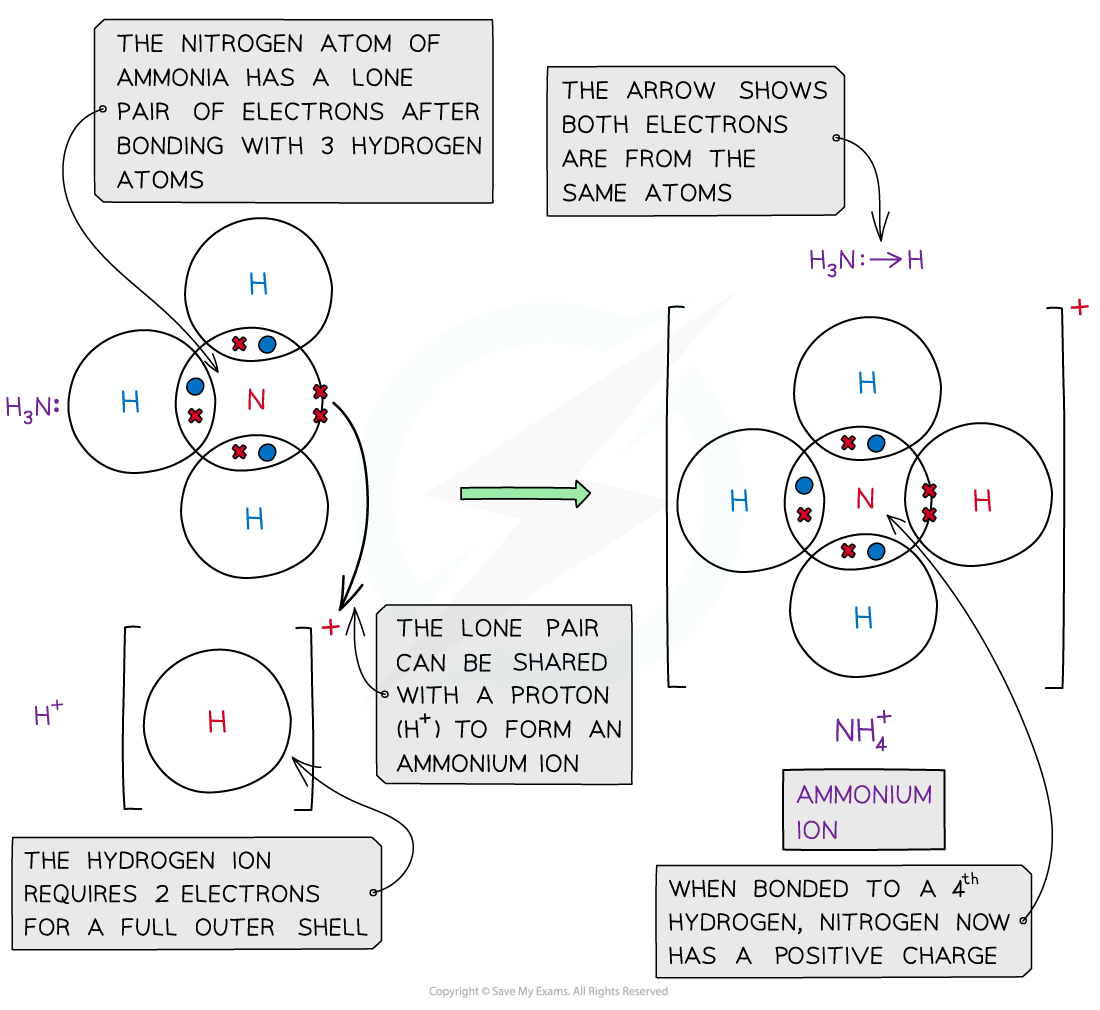
Ammonia (NH3) can donate a lone pair to an electron-deficient proton (H+) to form a charged ammonium ion (NH4+)
- Aluminium chloride is also formed using dative covalent bonding
- At high temperatures aluminium chloride can exist as a monomer (AlCl3)
- The molecule is electron-deficient and needs two electrons to complete the aluminium atom’s outer shell
- At lower temperatures the two molecules of AlCl3 join together to form a dimer (Al2Cl6)
- The molecules combine because lone pairs of electrons on two of the chlorine atoms form two coordinate bonds with the aluminium atoms
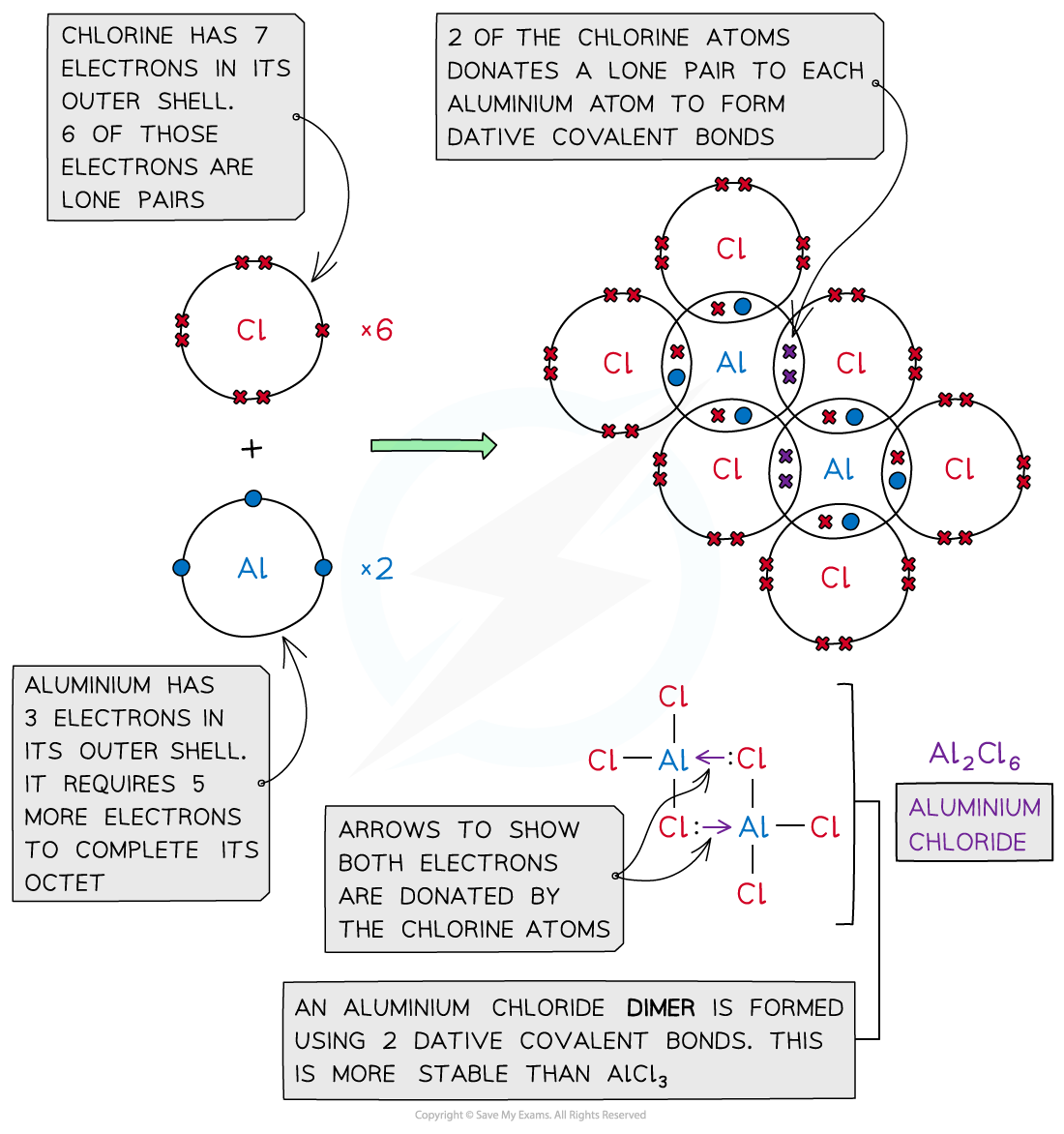
Aluminium chloride is also formed with a dative covalent bond in which two of the chlorine atoms donate their lone pairs to each of the aluminium atoms to form a dimer
Exam Tip
In dative covalent bonding, both electrons in the covalent bond are shared by one atom. A dative covalent bond is drawn using an arrow from the donated pair of electrons to the electron-deficient atom.
转载自savemyexams


最新发布
© 2025. All Rights Reserved. 沪ICP备2023009024号-1








