- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
AQA A Level Chemistry复习笔记1.4.2 Ionic Bonding
Ionic Bonding
- As a general rule, metals are on the left of the periodic table and nonmetals are on the right-hand side
- Ionic bonding involves the transfer of electrons from a metallic element to a non-metallic element
- Transferring electrons usually leaves the metal and the non-metal with a full outer shell
- Metals lose electrons from their valence shell forming positively charged cations
- Non-metal atoms gain electrons forming negatively charged anions
- Once the atoms become ions, their electronic configurations are the same as a noble gas
- A potassium ion (K+) has the same electronic configuration as argon: [2,8,8]+
- A chloride ion (Cl-) also has the same electronic configuration as argon: [2,8,8]-
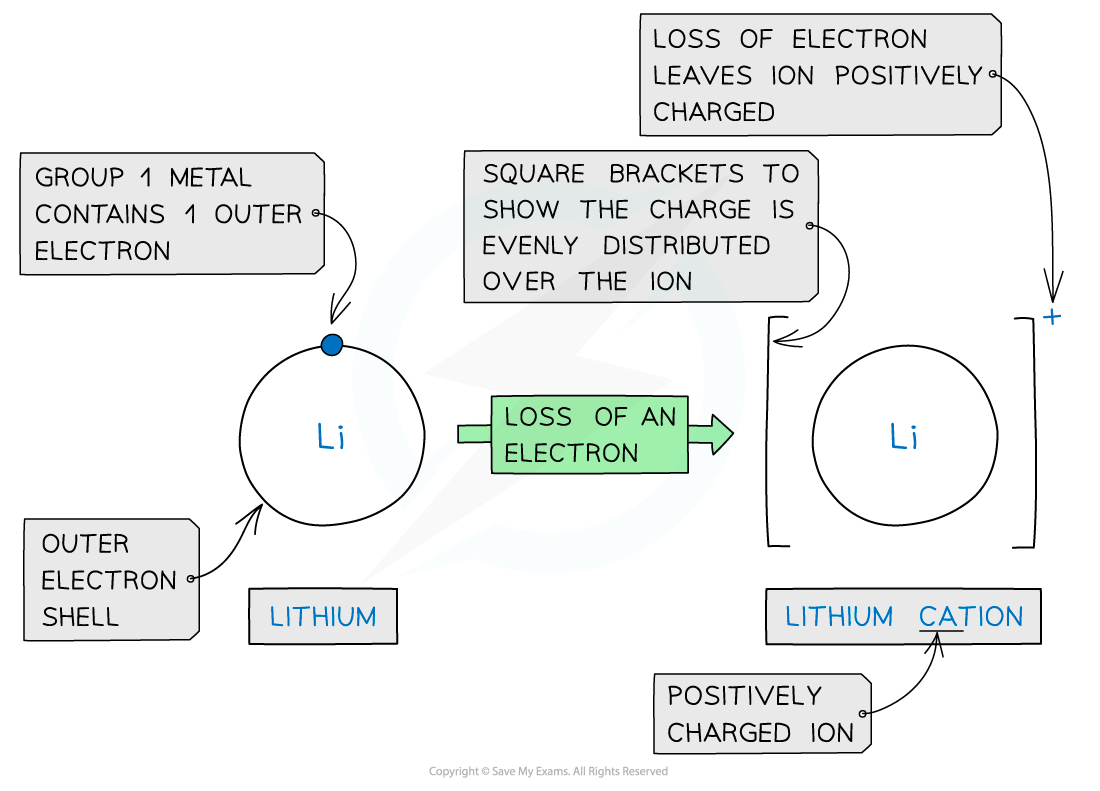
Forming cations by the removal of electrons from metals
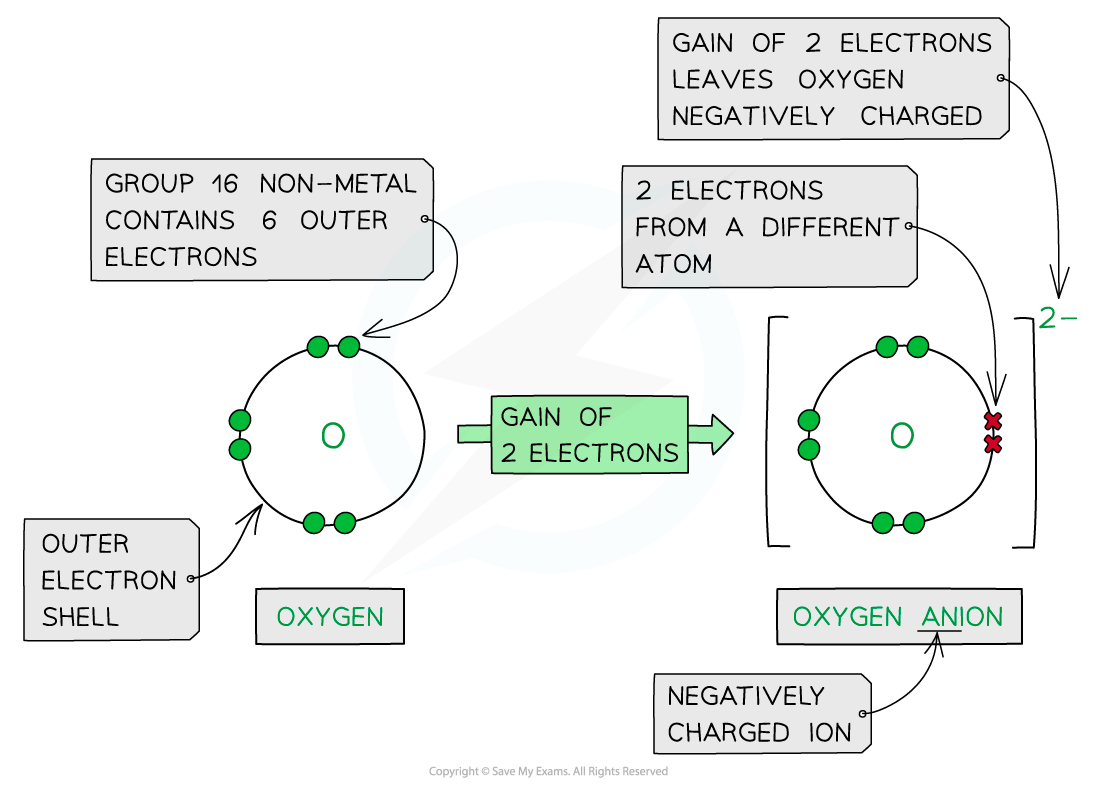
Forming anions by the addition of electrons to nonmetals
- Cations and anions are oppositely charged and therefore attracted to each other
- Electrostatic attractions are formed between the oppositely charged ions to form ionic compounds
- The ionic bond is the electrostatic attraction formed between the oppositely charged ions, which occurs in all directions ( this called non-directional bonding)
- This form of attraction is very strong and requires a lot of energy to overcome
- This causes high melting points in ionic compounds
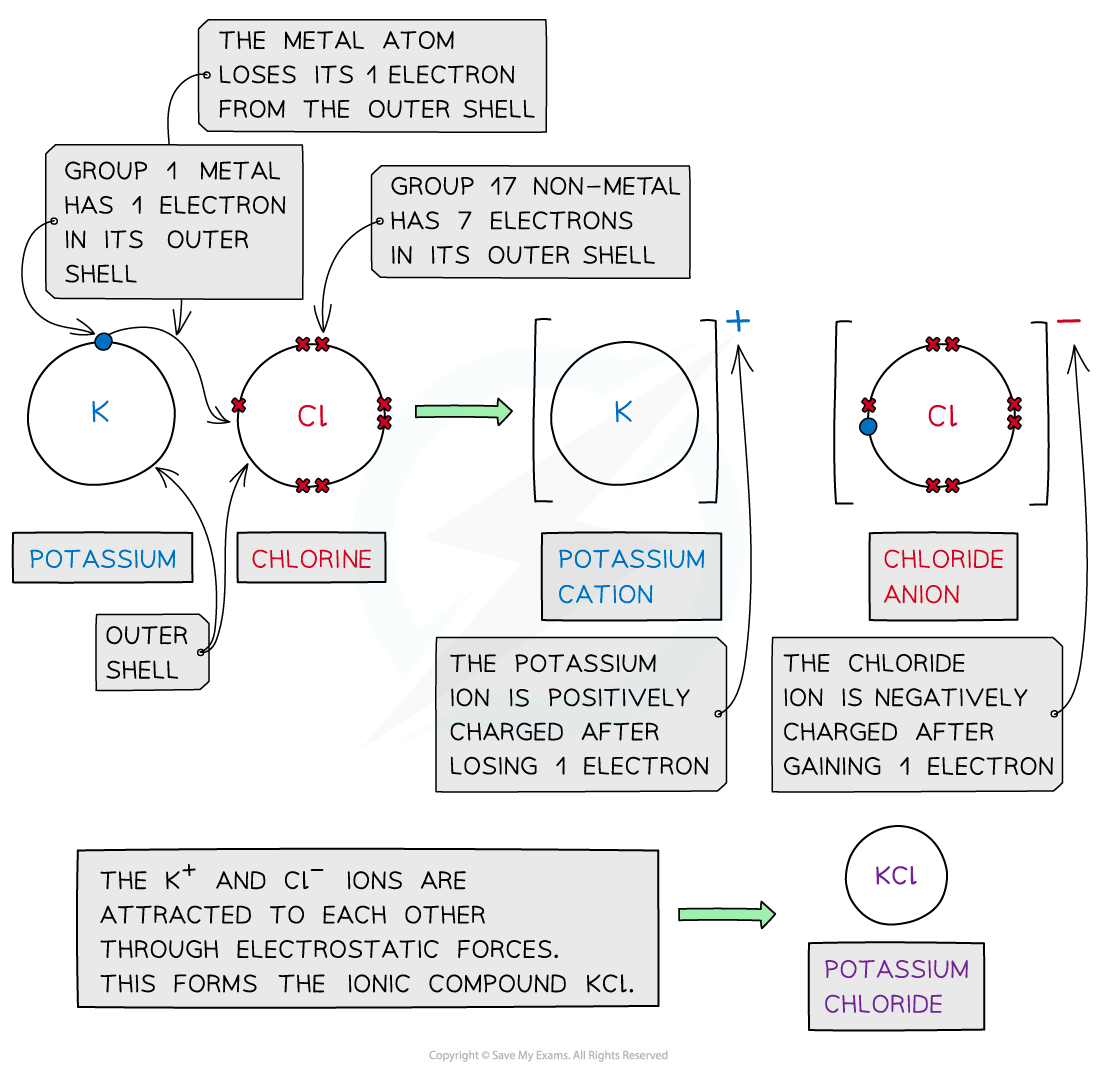 Cations and anions bond together using strong electrostatic forces, which require a lot of energy to overcome
Cations and anions bond together using strong electrostatic forces, which require a lot of energy to overcome
- The ions form a lattice structure which is an evenly distributed crystalline structure
- Ions in a lattice are arranged in a regular repeating pattern so that positive charges cancel out negative charges
- The attraction between the cations and anions is occurring in all directions
- Each ion is attracted to all of the oppositely charged ions around it
- Therefore the final lattice is overall electrically neutral
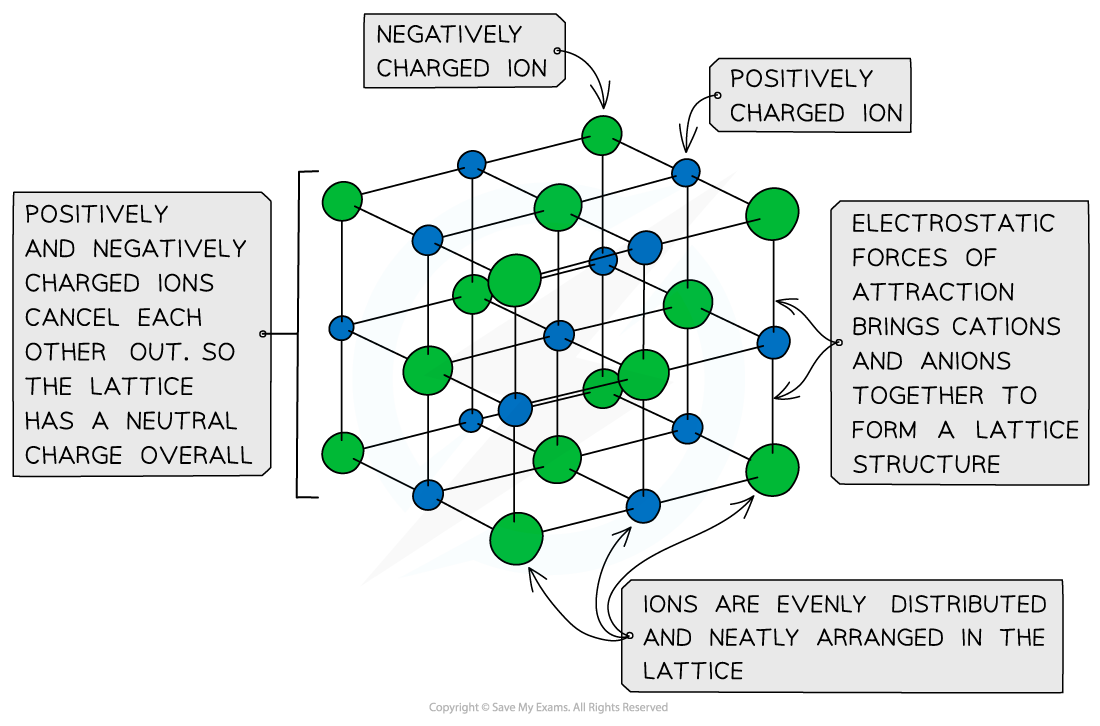
Ionic solids are arranged in lattice structures
Exam Tip
Metals usually lose all electrons from their outer valence shell to become cations.You can make use of the groups on the periodic table to work out how many electrons an atom is likely to lose or gain by looking at the group an atom belongs to.The electrostatic attraction between oppositely charged ions is the ionic bond.
转载自savemyexams


最新发布
© 2025. All Rights Reserved. 沪ICP备2023009024号-1








