- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
AQA A Level Chemistry复习笔记1.3.2 Reacting Masses
Reacting Masses
- The number of moles of a substance can be found by using the following equation:
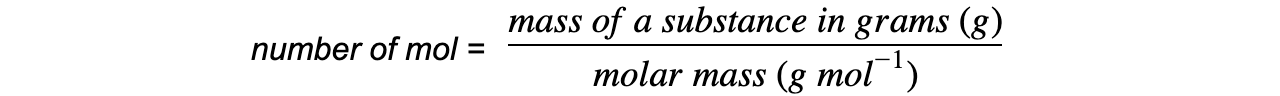
- It is important to be clear about the type of particle you are referring to when dealing with moles
- E.g. 1 mole of CaF2 contains one mole of CaF2 formula units, but one mole of Ca2+ and two moles of F- ions
Reacting masses
- The masses of reactants are useful to determine how much of the reactants exactly react with each other to prevent waste
- To calculate the reacting masses, the chemical equation is required
- This equation shows the ratio of moles of all the reactants and products, also called the stoichiometry, of the equation
- To find the mass of products formed in a reaction the following pieces of information are needed:
- The mass of the reactants
- The molar mass of the reactants
- The balanced equation
Worked Example
Mass calculation using molesCalculate the mass of magnesium oxide that can be made by completely burning 6 g of magnesium in oxygen.
magnesium (s) + oxygen (g) → magnesium oxide (s)
Answer
Step 1: The symbol equation is:
2Mg (s) + O2 (g) → 2MgO (s)
Step 2: The relative formula masses are:
Magnesium : 24 Oxygen : 32 Magnesium Oxide : 40
Step 3: Calculate the moles of magnesium used in reaction
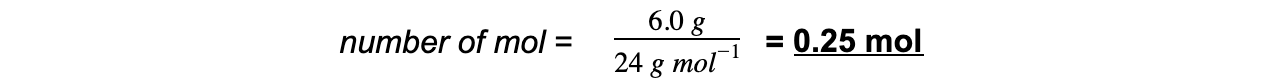
Step 4: Find the ratio of magnesium to magnesium oxide using the balanced chemical equation
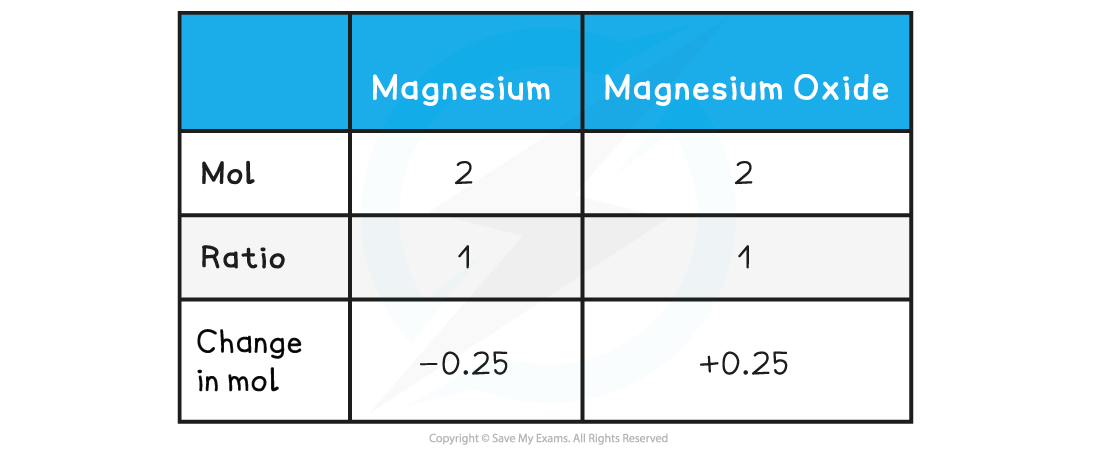
Therefore, 0.25 mol of MgO is formed
Step 5: Find the mass of magnesium oxide
mass = mol x Mr
mass = 0.25 mol x 40 g mol-1mass = 10 g
Therefore, mass of magnesium oxide produced is 10 g
Stoichiometric relationships
- The stoichiometry of a reaction can be found if the exact amounts of reactants and products formed are known
- The amounts can be found by using the following equation:
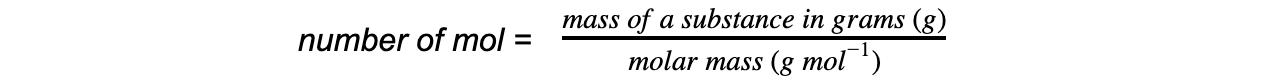
- The gas volumes can be used to deduce the stoichiometry of a reaction
- E.g. in the combustion of 50 cm3 of propane reacting with 250 cm3 of oxygen, 150 cm3 of carbon dioxide is formed suggesting that the ratio of propane:oxygen:carbon dioxide is 1:5:3
C3H8 (g) + 5O2 (g) → 3CO2 (g) + 4H2O (l)
转载自savemyexams

最新发布
© 2026. All Rights Reserved. 沪ICP备2023009024号-1









