- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
AQA A Level Chemistry复习笔记1.2.4 Reaction Yields
Percentage Yield
- In a lot of reactions, not all reactants react to form products which can be due to several factors:
- Other reactions take place simultaneously
- The reaction does not go to completion
- Reactants or products are lost to the atmosphere
- The percentage yield shows how much of a particular product you get from the reactants compared to the maximum theoretical amount that you can get:
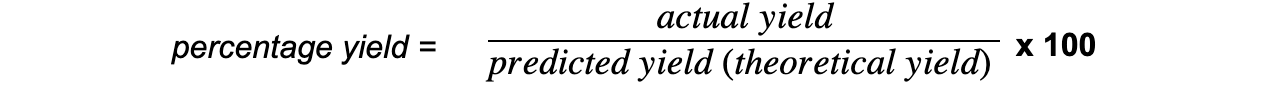
- The actual yield is the number of moles or mass of product obtained experimentally
- The predicted yield is the number of moles or mass obtained by calculation
- You will often have to use the following equation to work out the reacting masses, to calculate the predicted yield
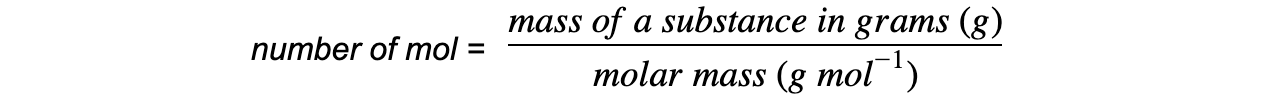
- It is important to be clear about the type of particle you are referring to when dealing with moles
- Eg. 1 mole of CaF2 contains one mole of CaF2 formula units, but one mole of Ca2+ and two moles of F- ions
Worked Example
Calculate % yield using molesIn an experiment to displace copper from copper sulfate, 6.5 g of zinc was added to an excess of copper (II) sulfate solution.The copper was filtered off, washed and dried.The mass of copper obtained was 4.8 g.Calculate the percentage yield of copper
Answer
Step 1: The symbol equation is:
Zn (s) + CuSO4 (aq) → ZnSO4 (aq) + Cu (s)
Step 2: Calculate the amount of zinc reacted in moles
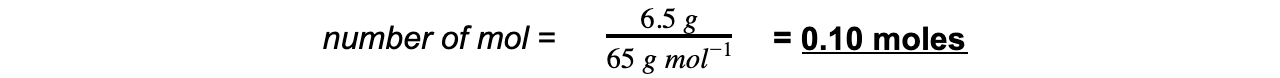
Step 3: Calculate the maximum amount of copper that could be formed from the molar ratio:
Since the ratio of Zn(s) to Cu(s) is 1:1 a maximum of 0.10 moles can be produced
Step 4: Calculate the maximum mass of copper that could be formed (theoretical yield)
mass = mol x Mr
mass = 0.10 mol x 64 g mol-1
mass = 6.4 gStep 5: Calculate the percentage yield of copper
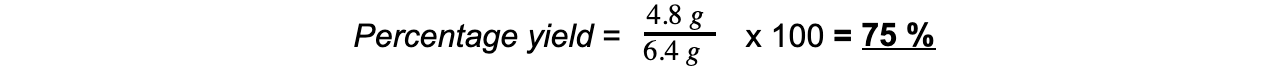
Limiting & Excess Reagents
Limiting & Excess reagents
- Sometimes, there is an excess of one or more of the reactants (excess reagent)
- The reactant which is not in excess is called the limiting reagent
- To determine which reactant is limiting:
- The number of moles of each reactant should be calculated
- The ratio of the reactants shown in the equation should be taken into account e.g.
2Na + S → Na2S
- Here, the ratio of Na : S is 2 : 1, and this should be taken into account when doing calculations
- Once all of one reactant has been used up, the reaction will stop, even if there are moles of the other reactant(s) leftover
- The reactant leftover is in excess, the reactant which causes the reaction to stop once it is used up is the limiting reagent
Worked Example
Excess & limiting reagent9.2 g of sodium is reacted with 8.0 g of sulfur to produce sodium sulfide, Na2S.Which reactant is in excess and which is the limiting reactant?
Answer
Step 1: Calculate the moles of each reactant
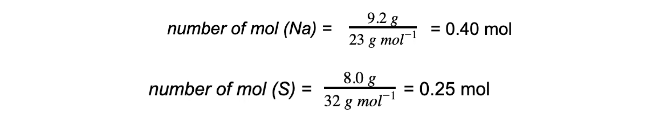
Step 2: Write the balanced equation and determine the molar ratio
2Na + S → Na2S
The molar ratio of Na: Na2S is 2:1
Step 3: Compare the moles and determine the limiting reagent
So to react completely 0.40 moles of Na require 0.20 moles of S and since there are 0.25 moles of S, then S is in excess. Na is therefore the limiting reactant.
Once all of the S has been used up, the reaction will stop, even though there is Na left.
转载自savemyexams

最新发布
© 2025. All Rights Reserved. 沪ICP备2023009024号-1









