- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
Edexcel IGCSE Biology 复习笔记 2.4.4 Practical: Osmosis
Edexcel IGCSE Biology 复习笔记 2.4.4 Practical: Osmosis
Practical: Factors that Influence Osmosis
- Osmosis is the diffusion of water molecules from a dilute solution (high concentration of water) to a more concentrated solution (low concentration of water) across a partially permeable membrane
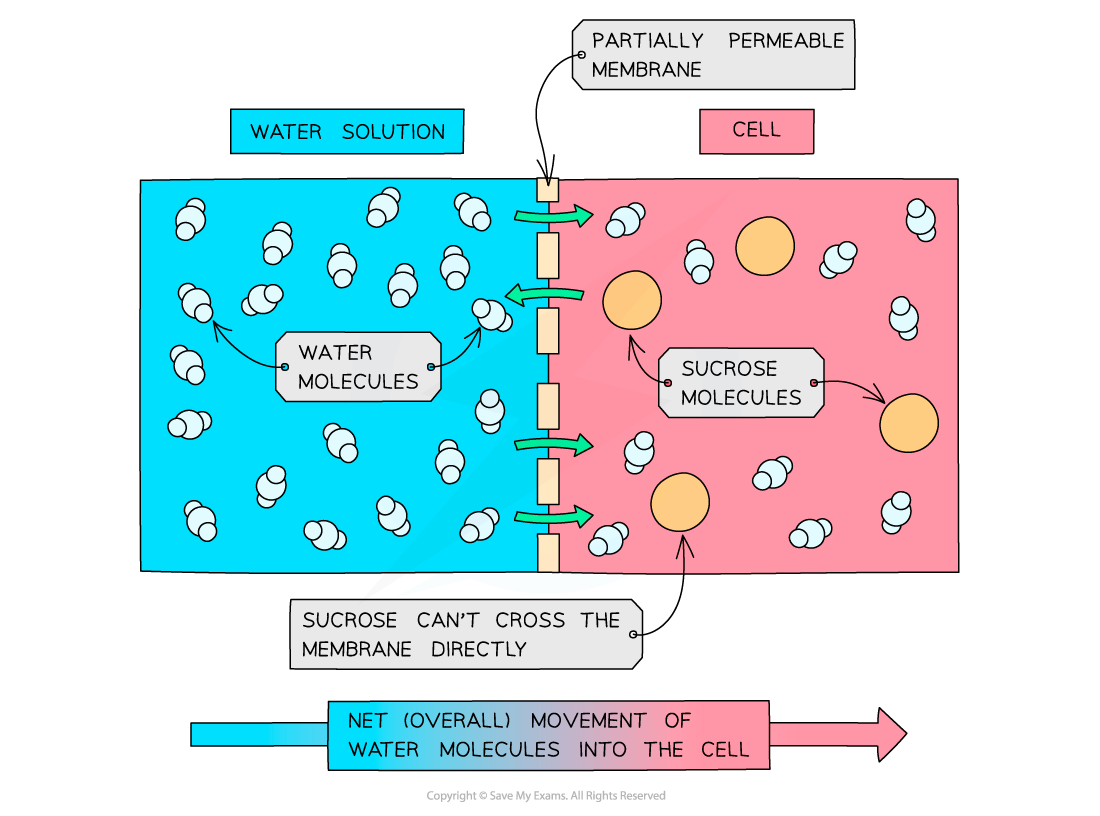 Osmosis in cells
Osmosis in cells
- We can investigate osmosis using cylinders of potato and placing them into distilled water and sucrose solutions of increasing concentration
Apparatus
- Potatoes
- Cork borer
- Knife
- Sucrose solutions (from 0 Mol/dm3 to 1 mol/dm3)
- Test tubes
- Balance
- Paper towels
- Ruler
- Test tube rack
Method
- Prepare a range of sucrose (sugar) solutions ranging from 0 Mol/dm3 (distilled water) to 1 mol/dm3
- Set up 6 labelled test tubes with 10cm3 of each of the sucrose solutions
- Using the knife, cork borer and ruler, cut 6 equally-sized cylinders of potato
- Blot each one with a paper towel and weigh on the balance
- Put 1 piece into each concentration of sucrose solution
- After 4 hours, remove them, blot with paper towels and reweigh them
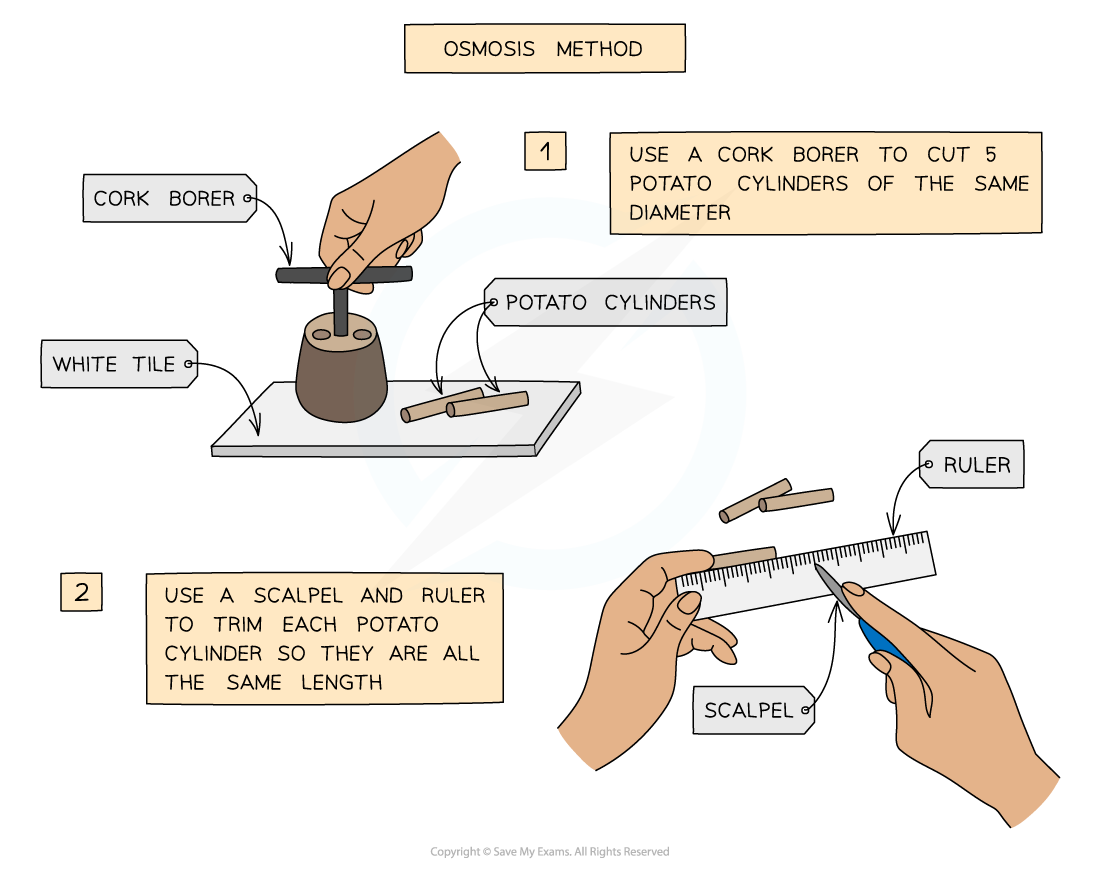
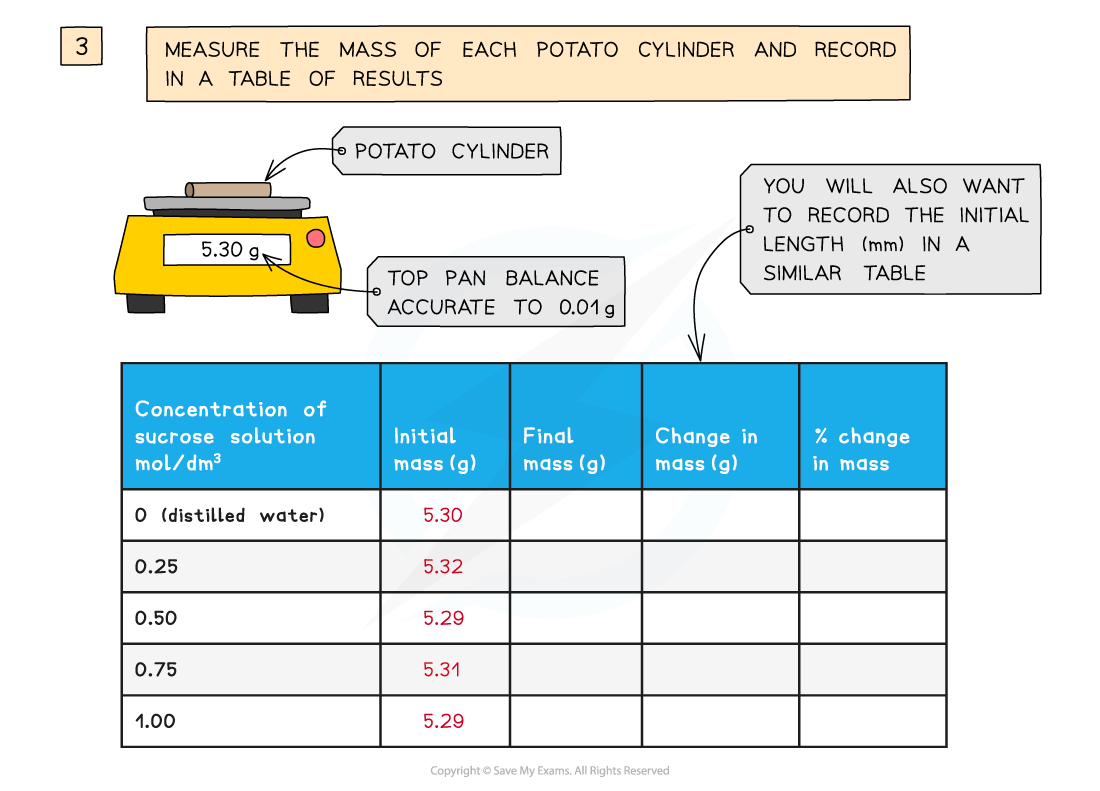
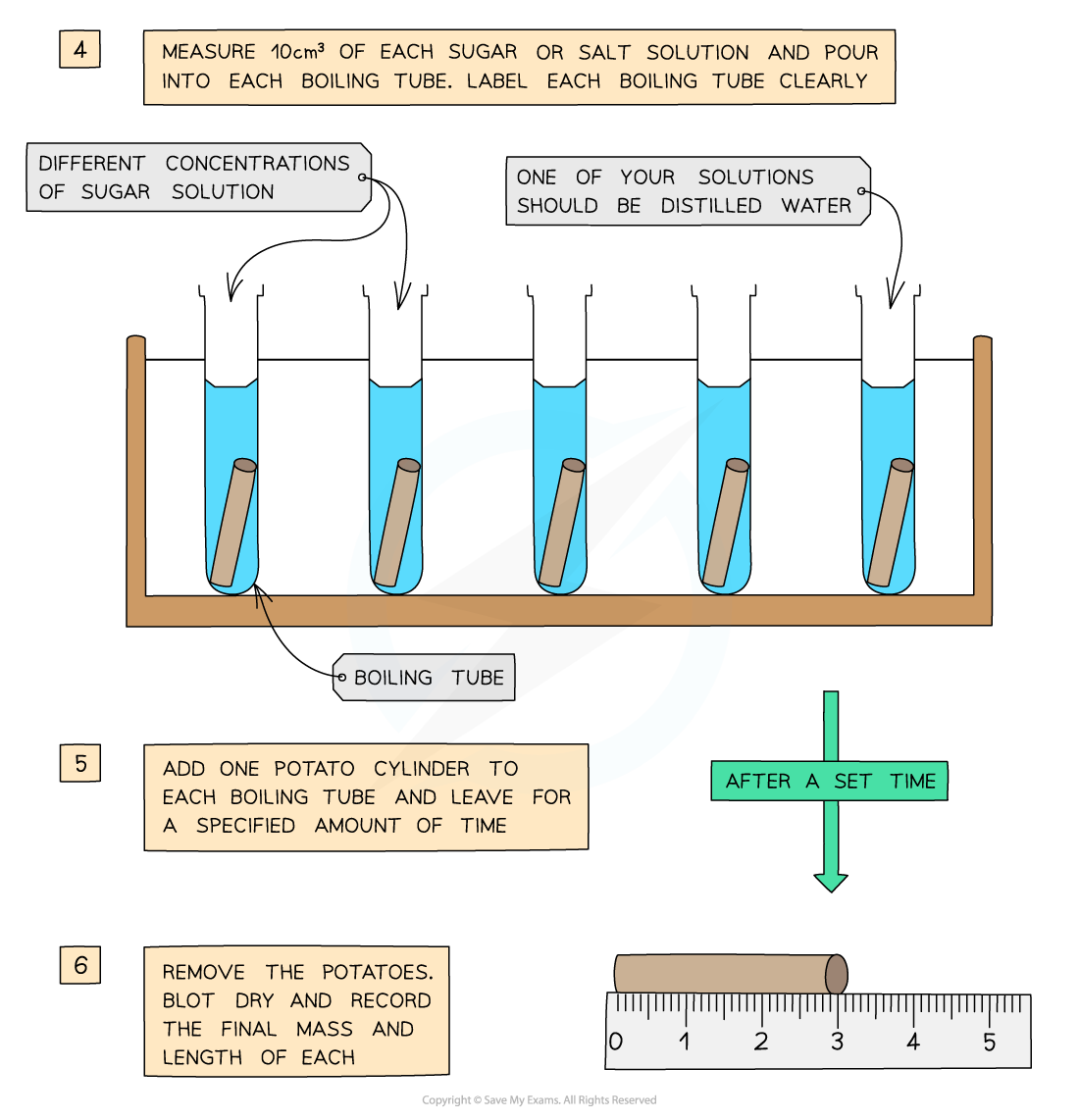 Experimental method for investigating osmosis in potato cylinders
Experimental method for investigating osmosis in potato cylinders
Results and analysis
- The percentage change in mass can be calculated for each piece of potato
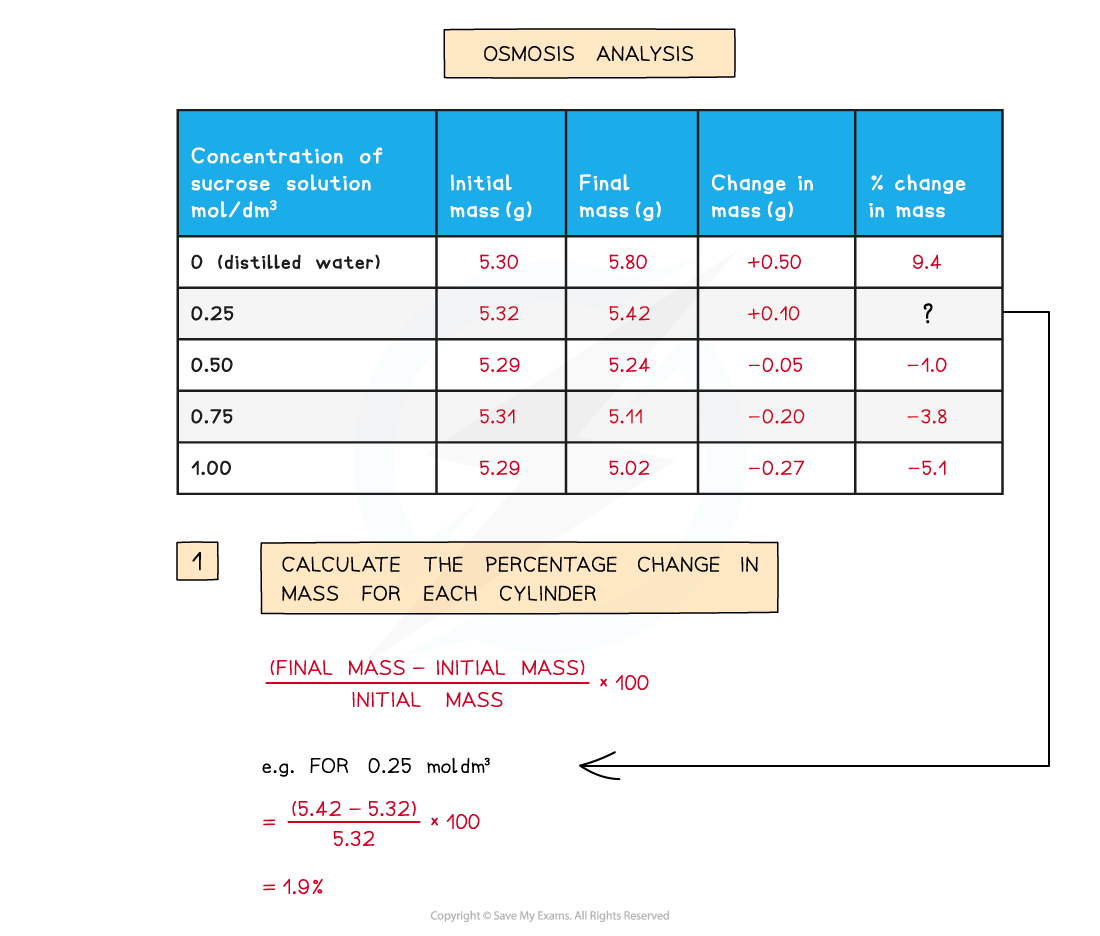 Calculating percentage change in mass
Calculating percentage change in mass
- The potato cylinder in the distilled water will have increased its mass the most as there is a greater concentration gradient in this tube between the distilled water (high water potential) and the potato cells (lower water potential)
- This means more water molecules will move into the potato cells by osmosis, pushing the cell membrane against the cell wall and so increasing the turgor pressure in the cells which makes them turgid - the potato cylinders will feel hard
- The potato cylinder in the strongest sucrose concentration will have decreased its mass the most as there is a greater concentration gradient in this tube between the potato cells (higher water potential) and the sucrose solution (lower water potential)
- This means more water molecules will move out of the potato cells by osmosis, making them flaccid and decreasing the mass of the cylinder - the potato cylinders will feel floppy
- If looked at underneath the microscope, cells from this potato cylinder might be plasmolysed, meaning the cell membrane has pulled away from the cell wall
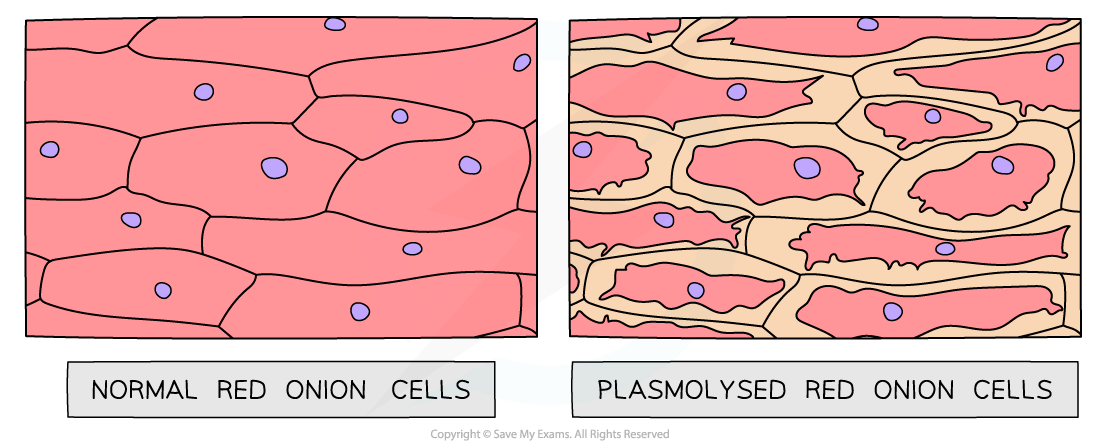 Plasmolysed red onion cells
Plasmolysed red onion cells
- If there is a potato cylinder that has not increased or decreased in mass, it means there was no overall net movement of water into or out of the potato cells
- This is because the solution that the cylinder was in was the same concentration as the solution found in the cytoplasm of the potato cells, so there was no concentration gradient
Limitations
- Slight differences in potato cylinders may mean that results aren't reliable or comparable
- Solution: for each sucrose concentration, repeat the investigation with several potato cylinders. Making a series of repeat experiments means that any anomalous results can be identified and ignored when a mean is calculated
Applying CORMS evaluation to practical work
- When working with practical investigations, remember to consider your CORMS evaluation
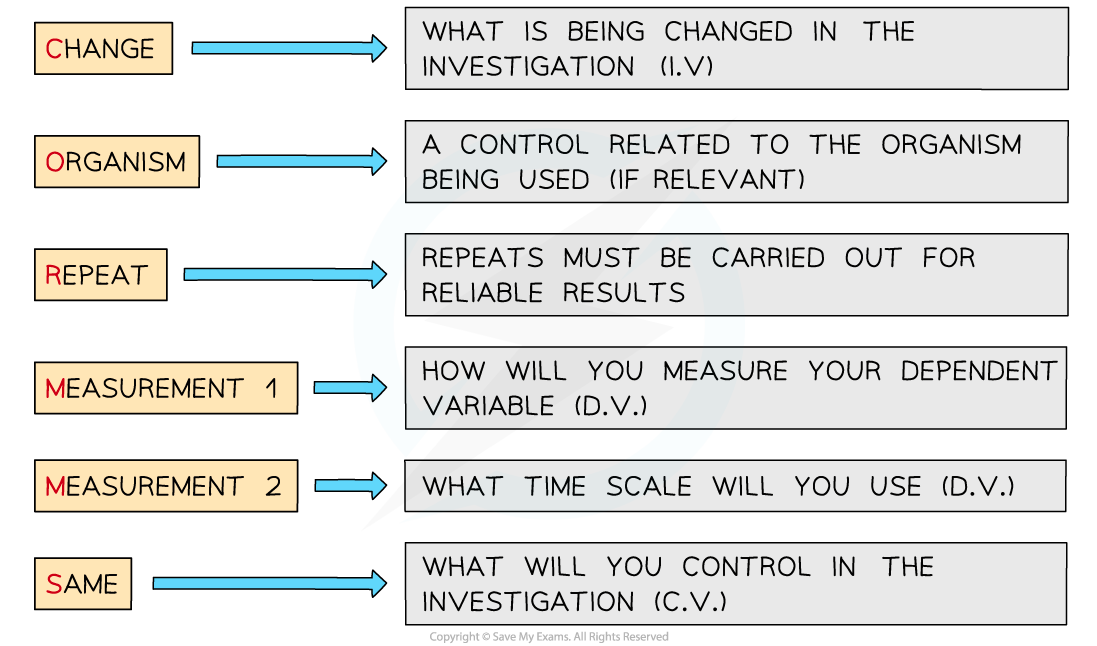 CORMS evaluation
CORMS evaluation
- In this investigation, your evaluation should look something like this:
- C - We are changing the concentration of sucrose solution
- O - The potato cylinders will all be taken from the same potato or potatoes of the same age
- R - We will repeat the investigation several times to ensure our results are reliable
- M1 - We will measure the change in mass of the potato cylinders
- M2 - ...after 4 hours
- S - We will control the volume of sucrose solution used, the dimensions of the potato cylinders and each cylinder must be blotted before it is weighed each time
Exam Tip
Questions involving osmosis experiments are common and you should be able to use your knowledge of these processes to explain the results.Don’t worry if it is an experiment you haven’t done – simply figure out where the higher concentration of water molecules is – this is the solution with the higher water potential - and explain which way the molecules move due to the differences in water potential.
转载自savemyexam

最新发布
© 2025. All Rights Reserved. 沪ICP备2023009024号-1









