- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
AQA A Level Biology复习笔记3.4.4 The Oxygen Dissociation Curve
The Oxygen Dissociation Curve
- The oxygen dissociation curve shows the rate at which oxygen associates, and also dissociates, with haemoglobin at different partial pressures of oxygen (pO2)
- Partial pressure of oxygen refers to the pressure exerted by oxygen within a mixture of gases; it is a measure of oxygen concentration
- Haemoglobin is referred to as being saturated when all of its oxygen binding sites are taken up with oxygen; so when it contains four oxygen molecules
- The ease with which haemoglobin binds and dissociates with oxygen can be described as its affinity for oxygen
- When haemoglobin has a high affinity it binds easily and dissociates slowly
- When haemoglobin has a low affinity for oxygen it binds slowly and dissociates easily
- In other liquids, such as water, we would expect oxygen to becomes associated with water, or to dissolve, at a constant rate, providing a straight line on a graph, but with haemoglobin oxygen binds at different rates as the pO2 changes; hence the resulting curve
- It can be said that haemoglobin's affinity for oxygen changes at different partial pressures of oxygen
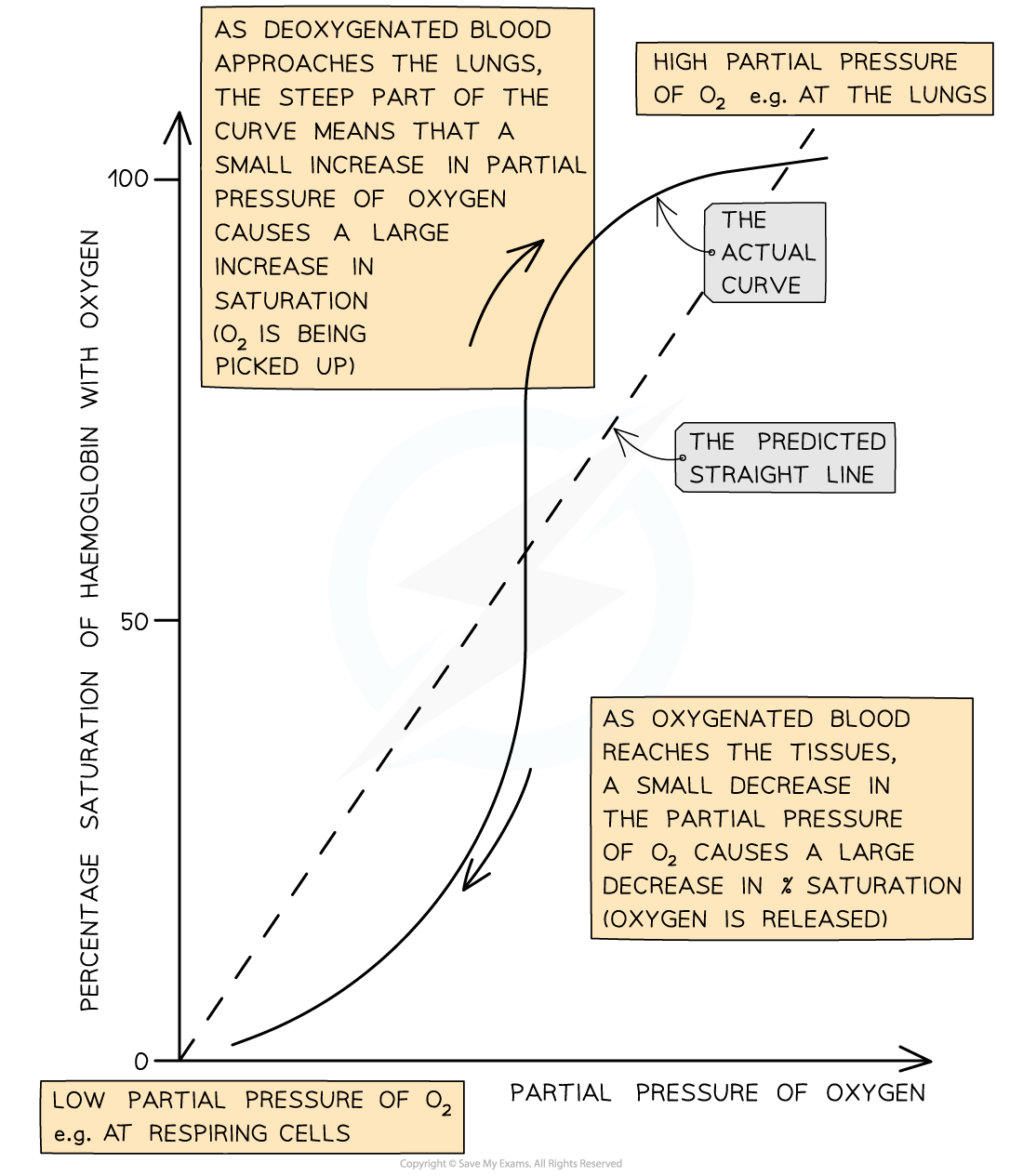
The oxygen dissociation curve shows the rate at which oxygen associates and dissociates with haemoglobin at different partial pressures of oxygen
Explaining the shape of the curve
- The curved shape of the oxygen dissociation curve for haemoglobin can be explained as follows
- Due to the shape of the haemoglobin molecule it is difficult for the first oxygen molecule to bind to haemoglobin; this means that binding of the first oxygen occurs slowly, explaining the relatively shallow curve at the bottom left corner of the graph
- After the first oxygen molecule binds to haemoglobin, the haemoglobin protein changes shape, or conformation, making it easier for the next haemoglobin molecules to bind; this speeds up binding of the remaining oxygen molecules and explains the steeper part of the curve in the middle of the graph
- The shape change of haemoglobin leading to easier oxygen binding is known as cooperative binding
- As the haemoglobin molecule approaches saturation it takes longer for the fourth oxygen molecule to bind due to the shortage of remaining binding sites, explaining the levelling off of the curve in the top right corner of the graph
Interpreting the curve
- When the curve is read from left to right, it provides information about the rate at which haemoglobin binds to oxygen at different partial pressures of oxygen
- At low pO2, in the bottom left corner of the graph, oxygen binds slowly to haemoglobin; this means that haemoglobin cannot pick up oxygen and become saturated as blood passes through the body's oxygen-depleted tissues
- Haemoglobin has a low affinity for oxygen at low pO2, so saturation percentage is low
- At medium pO2, in the central region of the graph, oxygen binds more easily to haemoglobin and saturation increases quickly; at this point on the graph a small increase in pO2 causes a large increase in haemoglobin saturation
- At high pO2, in the top right corner of the graph, oxygen binds easily to haemoglobin; this means that haemoglobin can pick up oxygen and become saturated as blood passes through the lungs
- Haemoglobin has a high affinity for oxygen at high pO2, so saturation percentage is high
- Note that at this point on the graph increasing the pO2 by a large amount only has a small effect on the percentage saturation of haemoglobin; this is because most oxygen binding sites on haemoglobin are already occupied
- At low pO2, in the bottom left corner of the graph, oxygen binds slowly to haemoglobin; this means that haemoglobin cannot pick up oxygen and become saturated as blood passes through the body's oxygen-depleted tissues
- When read from right to left, the curve provides information about the rate at which haemoglobin dissociates with oxygen at different partial pressures of oxygen
- In the lungs, where pO2 is high, there is very little dissociation of oxygen from haemoglobin
- At medium pO2, oxygen dissociates readily from haemoglobin, as shown by the steep region of the curve; this region corresponds with the partial pressures of oxygen present in the respiring tissues of the body, so ready release of oxygen is important for cellular respiration
- At this point on the graph a small decrease in pO2 causes a large decrease in percentage saturation of haemoglobin, leading to easy release of plenty of oxygen to the cells
- At low pO2 dissociation slows again; there are few oxygen molecules left on the binding sites, and the release of the final oxygen molecule becomes more difficult, in a similar way to the slow binding of the first oxygen molecule
转载自savemyexams

早鸟钜惠!翰林2025暑期班课上线

最新发布
© 2025. All Rights Reserved. 沪ICP备2023009024号-1








