- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
AQA A Level Biology复习笔记2.4.7 Osmosis in Animal Cells
Osmosis: Animal Cells
- Osmosis is the net movement of water molecules from a region of higher water potential (dilute solution) to a region of lower water potential (concentrated solution), through a partially permeable membrane
- Like plant cells, animal cells can also lose and gain water as a result of osmosis
- As animal cells do not have a supporting cell wall (unlike plant cells), the results of this loss or gain of water on the cell are more severe
- For example, if an animal cell is placed in a solution with a lower water potential than the cell (such as a concentrated sucrose solution), water will leave the cell through its partially permeable cell surface membrane by osmosis and the cell will shrink and shrivel up
- This occurs when the cell is in a hypertonic environment (the solution outside of the cell has a higher solute concentration than the inside of the cell)
- Conversely, if an animal cell is placed in pure water or a dilute solution, water will enter the cell through its partially permeable cell surface membrane by osmosis, as the pure water or dilute solution has a higher water potential. The cell will continue to gain water by osmosis until the cell membrane is stretched too far and the cell bursts (cytolysis), as it has no cell wall to withstand the increased pressure created
- This occurs when the cell is in a hypotonic environment (the solution outside of the cell has a lower solute concentration than the inside of the cell)
- This is why a constant water potential must be maintained inside the bodies of animals
- If an animal cell is in an isotonic environment (the solution outside of the cell has the same solute concentration as the inside of the cell), the movement of water molecules into and out of the cell occurs at the same rate (no net movement of water) and there is no change to the cells
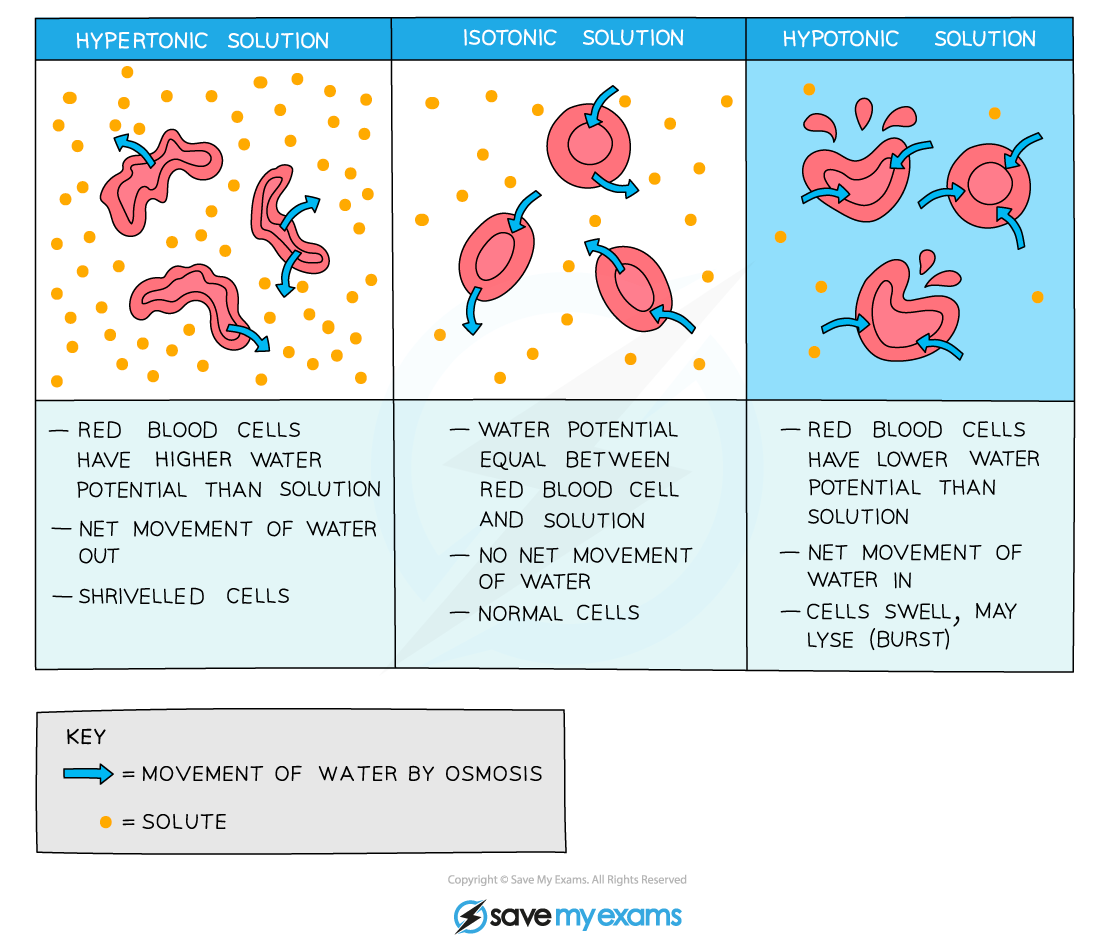
Effect of osmosis on animal cells
转载自savemyexams

早鸟钜惠!翰林2025暑期班课上线

最新发布
© 2025. All Rights Reserved. 沪ICP备2023009024号-1








