- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
AQA A Level Biology复习笔记1.6.3 The Properties of Water
Water in Cells
- Water is of great biological importance. It is the medium in which all metabolic reactions take place in cells. Between 70% to 95% of the mass of a cell is water
- As 71% of the Earth’s surface is covered in water it is a major habitat for organisms
- Water is composed of atoms of hydrogen and oxygen. One atom of oxygen combines with two atoms of hydrogen by sharing electrons (covalent bonding)
- Although water as a whole is electrically neutral the sharing of the electrons is uneven between the oxygen and hydrogen atoms
- The oxygen atom attracts the electrons more strongly than the hydrogen atoms, resulting in a weak negatively charged region on the oxygen atom (δ-) and a weak positively charged region on the hydrogen atoms(δ+), this also results in the asymmetrical shape
- This separation of charge due to the electrons in the covalent bonds being unevenly shared is called a dipole. When a molecule has one end that is negatively charged and one end that is positively charged it is also a polar molecule
- Water is a polar molecule
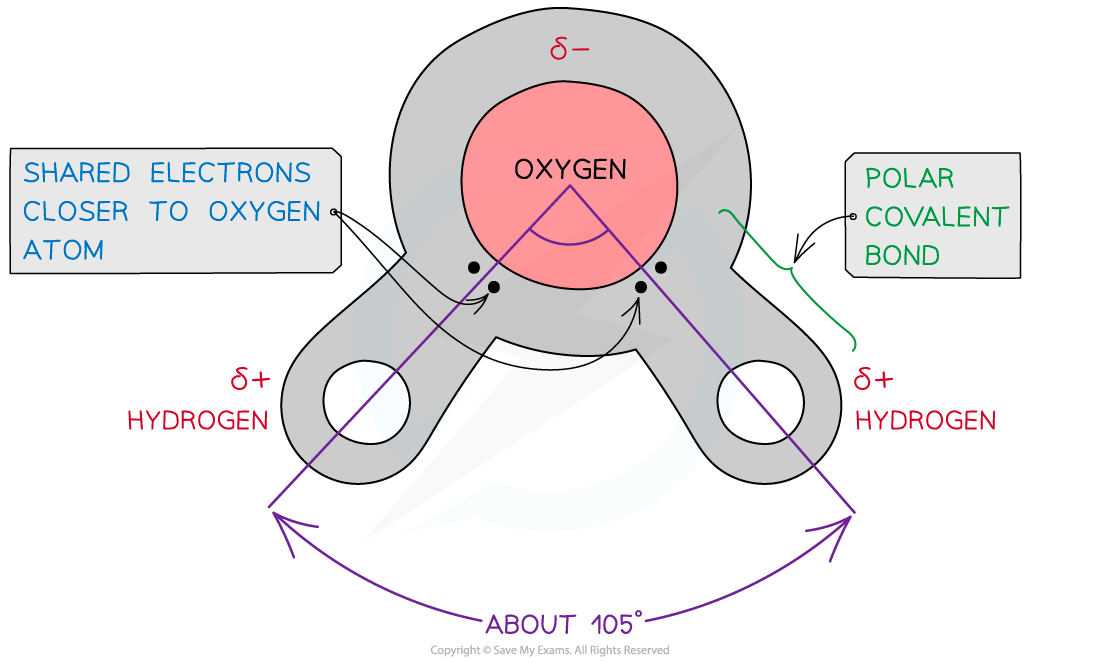
The covalent bonds of water make it a polar molecule
- Hydrogen bonds form between water molecules
- As a result of the polarity of water hydrogen bonds form between the positive and negatively charged regions of adjacent water molecules
- Hydrogen bonds are weak, when there are few, so they are constantly breaking and reforming. However when there are large numbers present they form a strong structure
- Hydrogen bonds contribute to the many properties water molecules have that make them so important to living organisms:
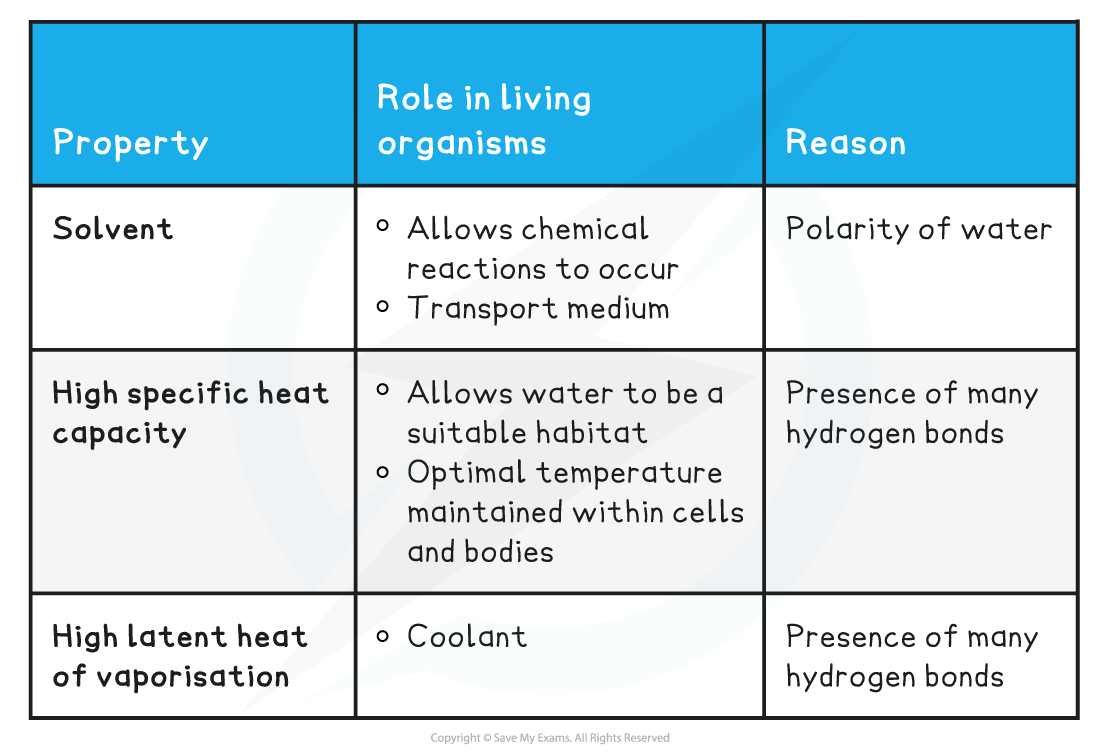
- An excellent solvent – many substances can dissolve in water
- A relatively high specific heat capacity
- A relatively high latent heat of vaporisation
- Water is less dense when a solid
- Water has high surface tension and cohesion
- It acts as a reagent
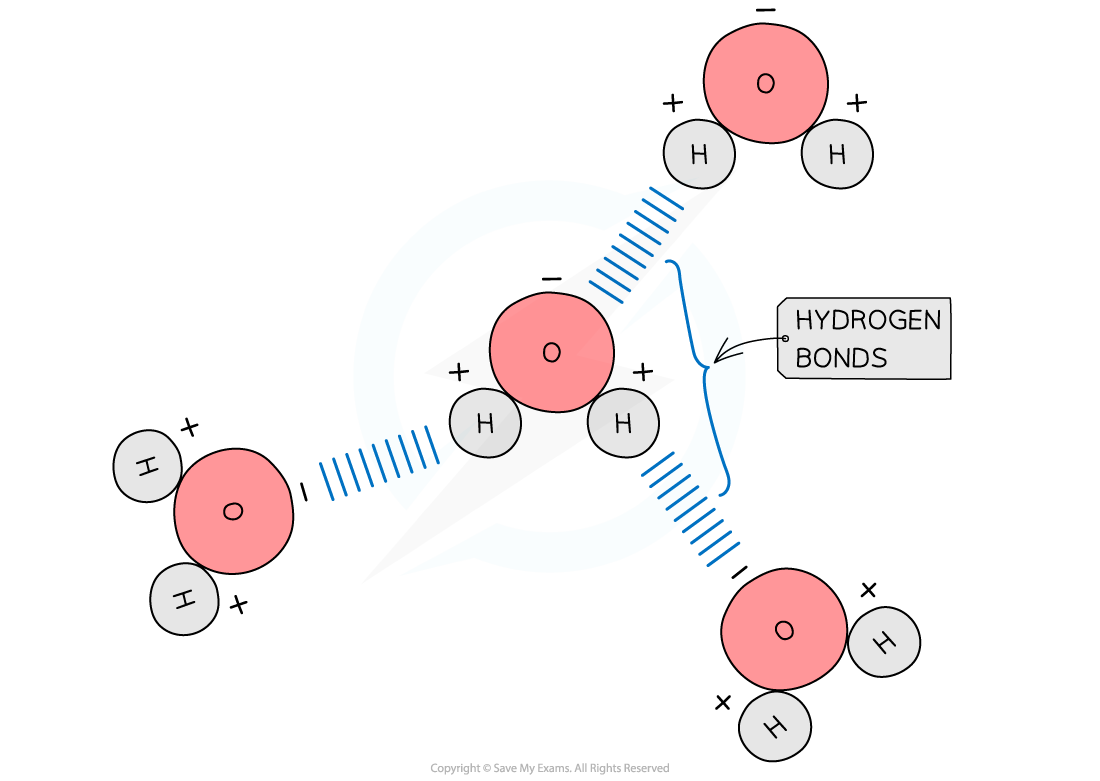
The polarity of water molecules allows hydrogen bonds to form between adjacent water molecules
- Water has many essential roles in living organisms due to its properties:
- The polarity of water molecules
- The presence and number of hydrogen bonds between water molecules
Solvent
- As water is a polar molecule many ions (e.g. sodium chloride) and covalently bonded polar substances (e.g. glucose) will dissolve in it
- This allows chemical reactions to occur within cells (as the dissolved solutes are more chemically reactive when they are free to move about)
- Metabolites can be transported efficiently (except non-polar molecules which are hydrophobic)
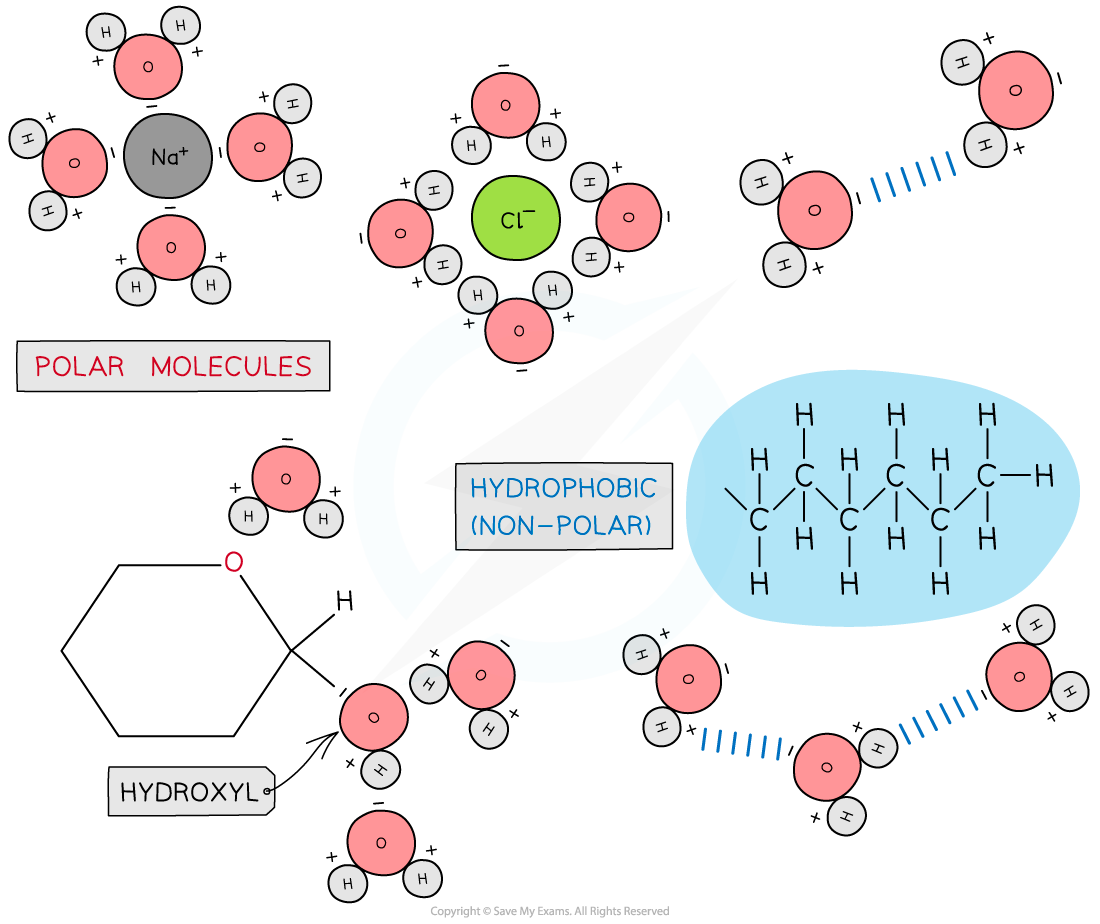
Due to its polarity water is considered a universal solvent
High specific heat capacity
- The specific heat capacity of a substance is the amount of thermal energy required to raise the temperature of 1kg of that substance by 1°C. Water’s specific heat capacity is 4200 J/kg°C
- Specific heat capacity is a measure of the energy required to raise the temperature of 1 kg of a substance by 1oC
- Water has a high specific heat capacity of 4200 J / Kg oC meaning a relatively large amount of energy is required to raise its temperature
- The high specific heat capacity is due to the many hydrogen bonds present in water. It takes a lot of thermal energy to break these bonds and a lot of energy to build them, thus the temperature of water does not fluctuate greatly
- The advantage for living organisms is that it:
- Provides suitable habitats
- Is able to maintain a constant temperature as water is able to absorb a lot of heat without big temperature fluctuations
- This is vital in maintaining temperatures that are optimal for enzyme activity
- Water in blood plasma is also vital in transferring heat around the body, helping to maintain a fairly constant temperature
- As blood passes through more active (‘warmer’) regions of the body, heat energy is absorbed but the temperature remains fairly constant
- Water in tissue fluid also plays an important regulatory role in maintaining a constant body temperature
Latent heat of vaporisation
- In order to change state (from liquid to gas) a large amount of thermal energy must be absorbed by water to break the hydrogen bonds and evaporate
- This is an advantage for living organisms as only a little water is required to evaporate for the organism to lose a great amount of heat
- This provides a cooling effect for living organisms, for example the transpiration from leaves or evaporation of water in sweat on the skin
Properties of Water & its Role in Living Organisms Table
Cohesion and adhesion
- Hydrogen bonds between water molecules allows for strong cohesion between water molecules
- This allows columns of water to move through the xylem of plants and through blood vessels in animals
- This also enables surface tension where a body of water meets the air, these hydrogen bonds occur between the top layer of water molecules to create a sort of film on the body of water (this is what allows insects such as pond skaters to float)
- Water is also able to hydrogen bond to other molecules, such as cellulose, which is known as adhesion
- This also enables water to move up the xylem due to transpiration
Exam Tip
It is important to know where the hydrogen bonds form between water molecules (oxygen of one water molecule to the hydrogen atom of another). Also, when discussing the role water has in living organisms remember to mention the ‘why’ in relation to its properties (ie. it is an excellent solvent due to the polar nature of water molecules).
转载自savemyexams

在线登记
最新发布
翰林课程体验,退费流程快速投诉邮箱: yuxi@linstitute.net 沪ICP备2023009024号-1








