- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
AQA A Level Biology复习笔记1.4.12 Limiting Factors Affecting Enzymes: Inhibitors
Enzyme Inhibitors
- An enzyme's activity can be reduced or stopped, temporarily, by a reversible inhibitor
- There are two types of reversible inhibitors:
- Competitive inhibitors have a similar shape to that of the substrate molecules and therefore compete with the substrate for the active site
- Non-competitive inhibitors bind to the enzyme at an alternative site, which alters the shape of the active site and therefore prevents the substrate from binding to it
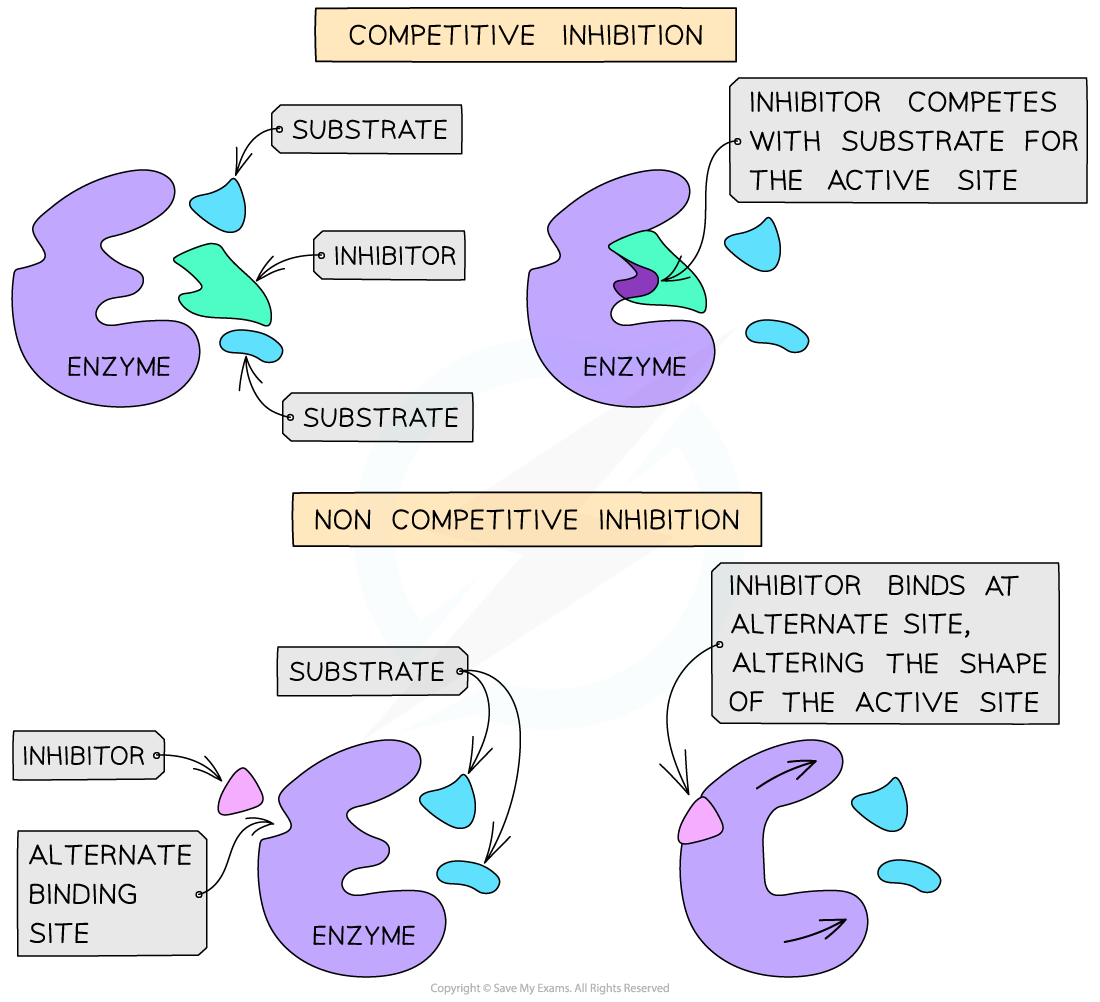
Competitive and non-competitive inhibition
- Reversible inhibitors can act as regulators in metabolic pathways
- Metabolic reactions must be very tightly controlled and balanced, so that no single enzyme can ‘run wild’ and continuously and uncontrollably generate more and more of a particular product
- Metabolic reactions can be controlled by using the end-product of a particular sequence of metabolic reactions as a non-competitive, reversible inhibitor:
- As the enzyme converts substrate to product, the process is itself slowed down as the end-product of the reaction chain binds to an alternative site on the original enzyme, changing the shape of the active site and preventing the formation of further enzyme-substrate complexes
- The end-product can then detach from the enzyme and be used elsewhere, allowing the active site to reform and the enzyme to return to an active state
- This means that as product levels fall, the enzyme begins catalysing the reaction once again, in a continuous feedback loop
- This process is known as end-product inhibition
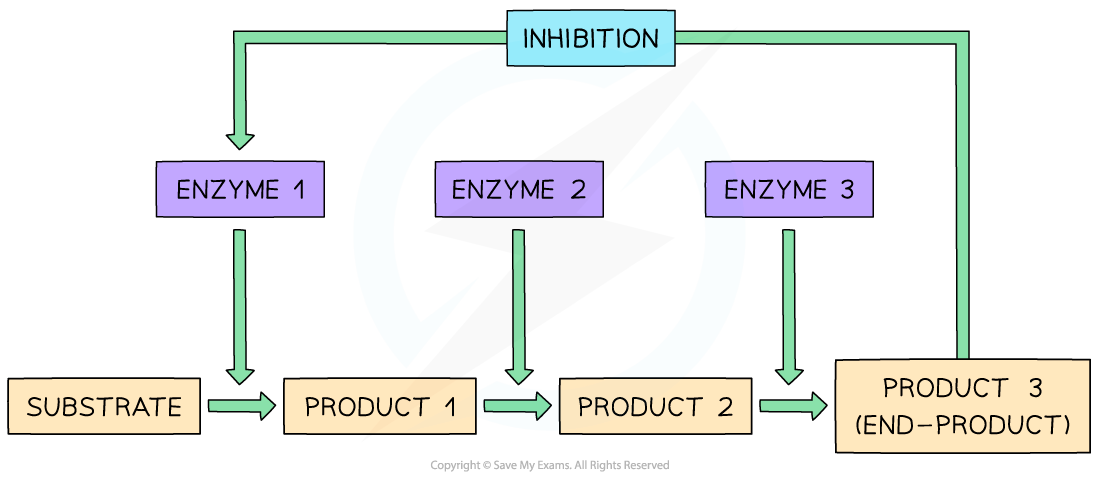
End-product inhibition
Rate: Inhibitor Concentration
- There are two types of inhibitors:
- Competitive inhibitors have a similar shape to that of the substrate molecules and therefore compete with the substrate for the active site
- Non-competitive inhibitors bind to the enzyme at an alternative site, which alters the shape of the active site and therefore prevents the substrate from binding to it
- Both types of inhibitors slow down or stop enzyme activity
- Increasing the concentration of an inhibitor, therefore, reduces the rate of reaction and eventually, if inhibitor concentration continues to be increased, the reaction will stop completely
- For competitive inhibitors, countering the increase in inhibitor concentration by increasing the substrate concentration can increase the rate of reaction once more (more substrate molecules mean they are more likely to collide with enzymes and form enzyme-substrate complexes)
- For non-competitive inhibitors, increasing the substrate concentration cannot increase the rate of reaction once more, as the shape of the active site of the enzyme remains changed and enzyme-substrate complexes are still unable to form
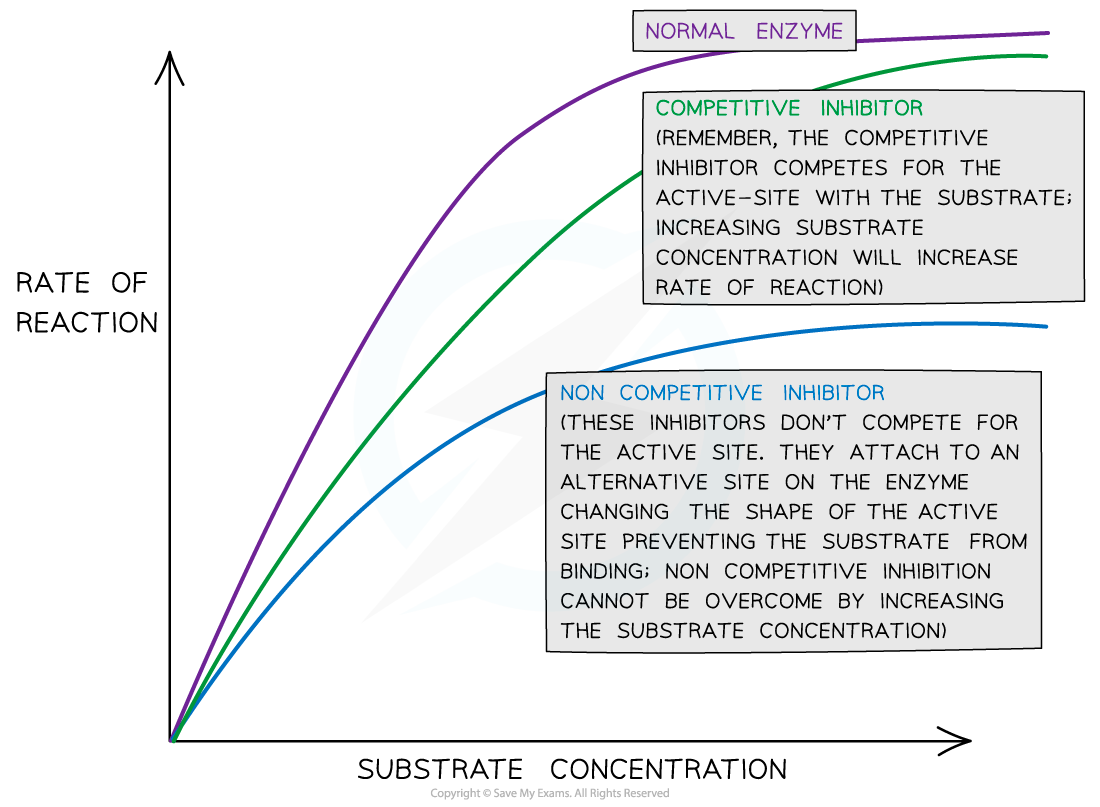
The effect of inhibitor concentration on the rate of an enzyme-catalysed reaction
转载自savemyexams

最新发布
© 2025. All Rights Reserved. 沪ICP备2023009024号-1









