- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
AQA A Level Biology复习笔记1.1.10 Biochemical Tests: Sugars & Starch
Biochemical Tests: Sugars & Starch
- There are a number of tests that can be carried out quickly and easily in a lab to determine if a sample contains a certain type of sugar
- The following tests are qualitative - they do not give a quantitative value as to how much of each type of molecule may be present in a sample
- Sugars can be classified as reducing or non-reducing; this classification is dependent on their ability to donate electrons (a reducing sugar that is able to donate electrons is itself oxidised)
- OILRIG in Chemistry
The Benedict’s test for reducing sugars
- Benedict’s reagent is a blue solution that contains copper (II) sulfate ions (CuSO4 ); in the presence of a reducing sugar copper (I) oxide forms
- Copper (I) oxide is not soluble in water, so it forms a precipitate
Method
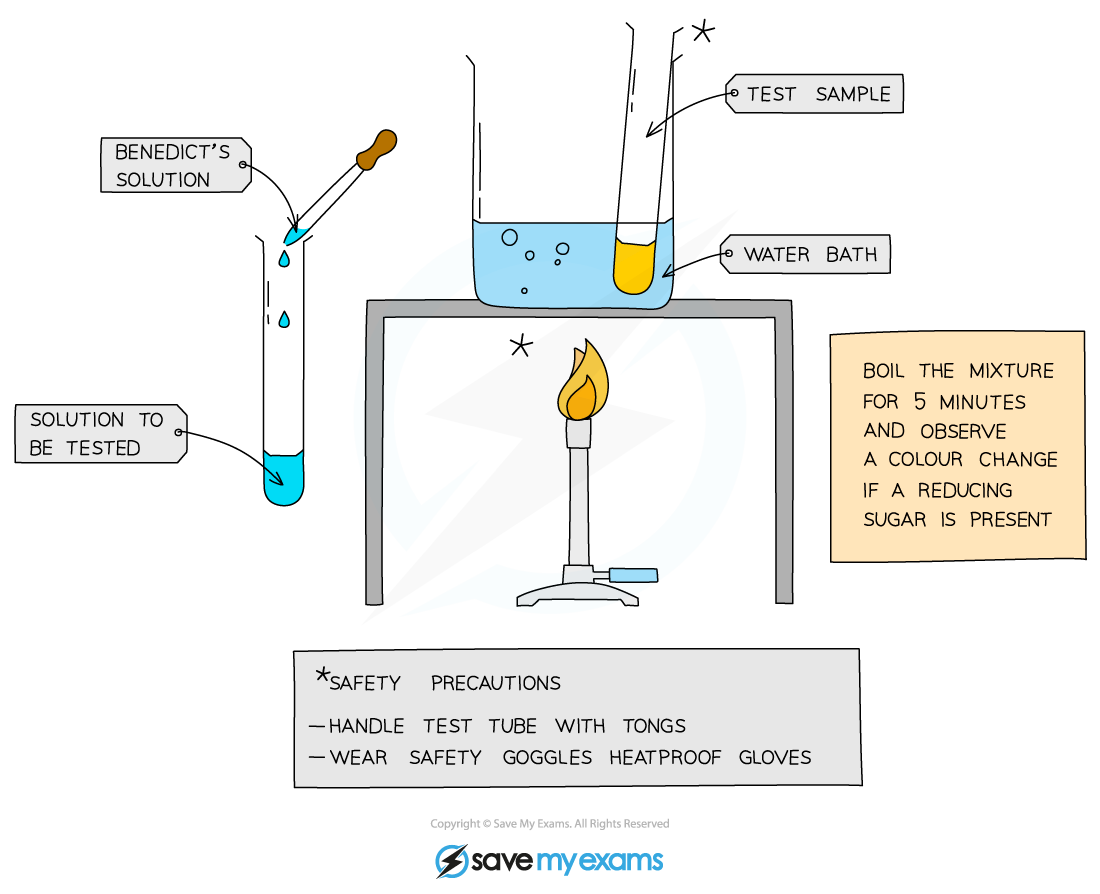
- Add Benedict's reagent (which is blue as it contains copper (II) sulfate ions) to a sample solution in a test tube
- Heat the test tube in a water bath or beaker of water that has been brought to a boil for a few minutes
- If a reducing sugar is present, a coloured precipitate will form as copper (II) sulfate is reduced to copper (I) oxide which is insoluble in water
- It is important that an excess of Benedict’s solution is used so that there is more than enough copper (II) sulfate present to react with any sugar present
- A positive test result is a colour change somewhere along a colour scale from blue (no reducing sugar), through green, yellow and orange (low to medium concentration of reducing sugar) to brown/brick-red (a high concentration of reducing sugar)
- This test is semi-quantitative as the degree of the colour change can give an indication of how much (the concentration of) reducing sugar present
The Benedict's test for reducing sugars produces a colour change from blue towards red if a reducing sugar is present
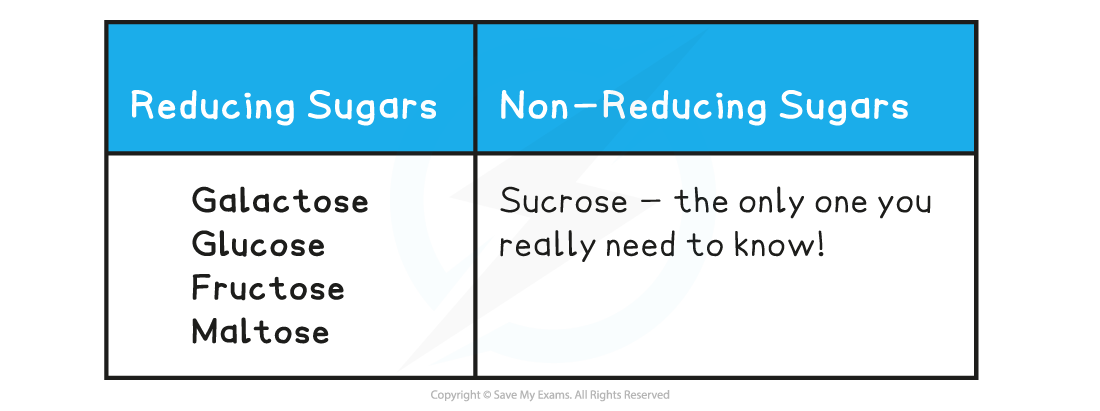
Reducing & Non-reducing Sugars Table
To test for non-reducing sugars:
- Add dilute hydrochloric acid to the sample and heat in a water bath that has been brought to the boil
- Neutralise the solution with sodium hydrogencarbonate
- Use a suitable indicator (such as red litmus paper) to identify when the solution has been neutralised, and then add a little more sodium hydrogencarbonate as the conditions need to be slightly alkaline for the Benedict’s test to work
- Then carry out the Benedict’s test as normal; add Benedict’s reagent to the sample and heat in a water bath that has been boiled – if a colour change occurs, a reducing sugar is present
Explanation
- The addition of acid will hydrolyse any glycosidic bonds present in any carbohydrate molecules
- The resulting monosaccharides left will have an aldehyde or ketone functional group that can donate electrons to copper (II) sulfate (reducing the copper), allowing a precipitate to form
The iodine test for starch
- To test for the presence of starch in a sample, add a few drops of orange/brown iodine in potassium iodide solution to the sample
- The iodine is in potassium iodide solution as iodine is insoluble in water
- If starch is present, iodide ions in the solution interact with the centre of starch molecules, producing a complex with a distinctive blue-black colour
- This test is useful in experiments for showing that starch in a sample has been digested by enzymes
转载自savemyexams

早鸟钜惠!翰林2025暑期班课上线

最新发布
© 2025. All Rights Reserved. 沪ICP备2023009024号-1








