- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
AQA A Level Chemistry复习笔记7.2.1 Aldehydes & Ketones
Oxidation of Aldehydes
- Aldehydes and ketones contain the carbonyl functional group, C=O
- This is why aldehydes and ketones are also known as carbonyls
- The difference between aldehydes and ketones is the groups bonded to the carbon of the carbonyl group

- The carbonyl group in an aldehyde is always situated at the end of the chain
- When naming aldehydes, you do not include the '1' in the name, the carbonyl carbon is always number 1 on the chain
- The simplest aldehyde is methanal, HCHO, with the only carbon being that of the carbonyl group
- The carbonyl group in a ketone is always situated in the middle of the chain
- The simplest ketone is propan-2-one, CH3COCH3, as you need an alkyl group either side of the carbonyl carbon in a ketone
Preparation of Aldehydes & Ketones
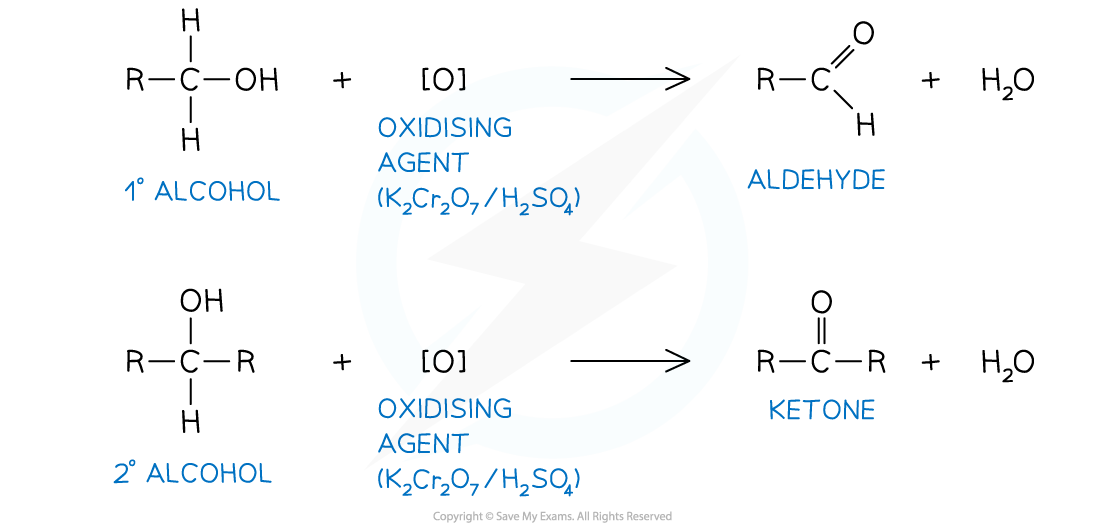
- Aldehydes and ketones can be prepared by oxidising primary and secondary alcohols as shown below
Further Oxidation
- During the oxidation of a primary alcohol to an aldehyde, the apparatus must be set up to distill off the aldehyde as it is produced
- Further oxidation of primary alcohols can then take place
- Aldehydes can be easily oxidised to form carboxylic acids
- To oxidise a primary alcohol straight to a carboxylic acid, you would heat the reaction mixture under reflux
- The aldehyde would still be produced, but as it evaporates it would condense and drop back into the reaction mixture, to be further oxidised to the carboxylic acid
- The oxidising agent used for all of the oxidation reactions be acidified potassium dichromate
- K2Cr2O7 with sulfuric acid, H2SO4
- Ketones are very resistant to being oxidised, so no further oxidation reaction will take place with secondary alcohols
- This is because ketones do not have a readily available hydrogen atom, in the same way that aldehydes (or alcohols) do
- An extremely strong oxidising agent would be needed for oxidation of a ketone to take place
- The oxidation will likely oxidise a ketone in a destructive way, breaking a C-C bond

Exam Tip
In the exam you can simply say that ketones cannot be oxidised!
Distinguishing Between Aldehydes & Ketones
Distinguishing Between Aldehydes & Ketones
- Weak oxidising agents can be used to distinguish between an aldehyde and a ketone
- The aldehyde will be oxidised to a carboxylic acid, but the ketone will not undergo oxidation
- There are a number of tests that can be used to distinguish between aldehydes and ketones
- You specifically need to know the following methods to distinguish between an aldehyde and a ketone:
- Tollens' reagent (this is the most commonly used method)
- Fehling's solution
Using Tollens' Reagent - The Silver Mirror Test
- Tollens' reagent contains the silver(I) complex ion [Ag(NH3)2]+
- This is formed when aqueous ammonia is added to a solution of silver nitrate
- Tollens' reagent is also known as ammoniacal silver nitrate
- If gently warmed with Tollens' reagent, an aldehyde will become oxidised
- The silver(I) complex ion solution, [Ag(NH3)2]+, is colourless
- As the aldehyde is oxidised, it causes the [Ag(NH3)2]+ ions to become reduced to solid metallic silver, Ag
- This is why a positive test result is called a "silver mirror"
Positive Test Result:
- When Tollens' reagent is gently warmed with an aldehyde, the silver mirror is formed
- This is the positive test result
- When Tollens' reagent is gently warmed with a ketone, no silver mirror will be seen, as the ketone cannot be oxidised by Tollens' reagent, so no reaction takes place
- This is a negative test result
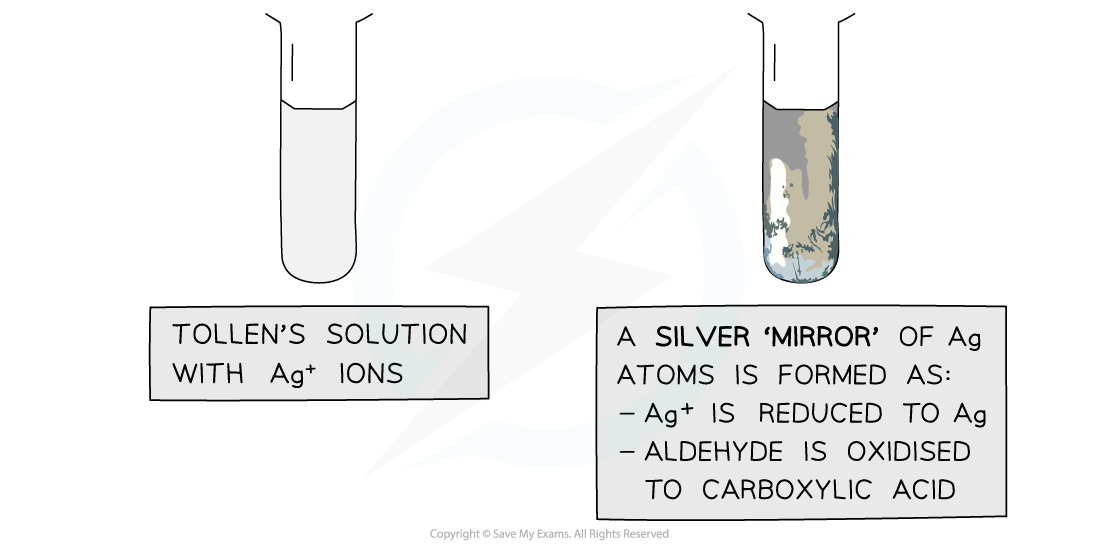
The Ag+ ions in Tollens’ reagent are oxidising agents, oxidising the aldehyde to a carboxylic acid and getting reduced themselves to silver atoms
Using Fehling's Solution
- Fehling’s solution is an alkaline solution containing copper(II) ions which act as the oxidising agent
- If an aldehyde is warmed with Fehling's solution, the aldehyde will be oxidised and a colour change will take place
- Fehling's solution is blue, because of the copper(II) complex ions present
- During the reaction, as the aldehyde is oxidised to a carboxylic acid, the blue Cu2+ ions are reduced to Cu+ ions and a brick red precipitate is formed
- The brick red precipitate is copper(I) oxide
- If a ketone is warmed with Fehling's solution, no reaction takes place as the ketone will not be oxidised, so the solution will remain blue
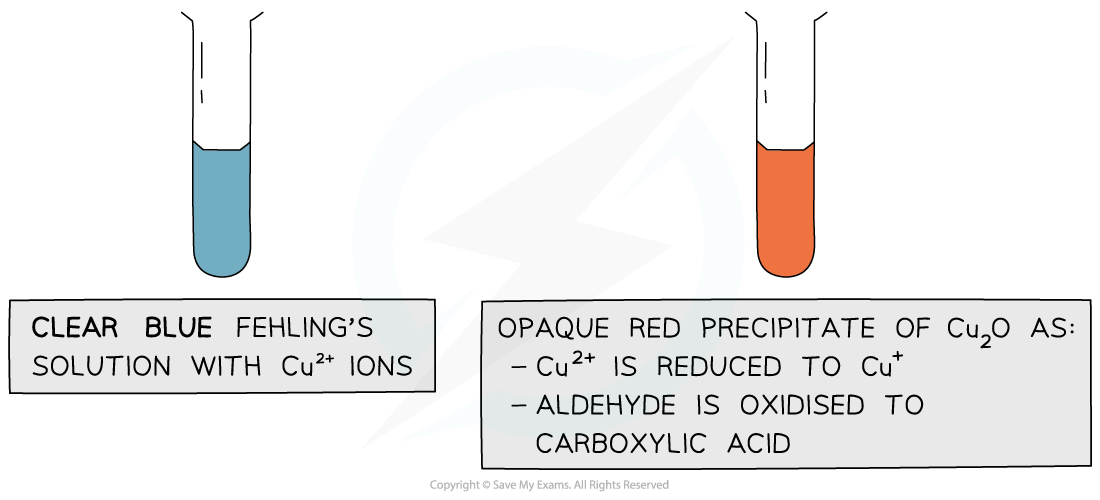
The copper(II) ions in Fehling’s solution are oxidising agents, oxidising the aldehyde to a carboxylic acid and getting reduced themselves to copper(I) ions in the Cu2O precipitate
- Heating with acidified potassium dichromate could also be used to distinguish between an aldehyde and a ketone
- The aldehyde would be oxidised, and you would see an orange to green colour change
- The ketone would not be oxidised, so you would see no colour change
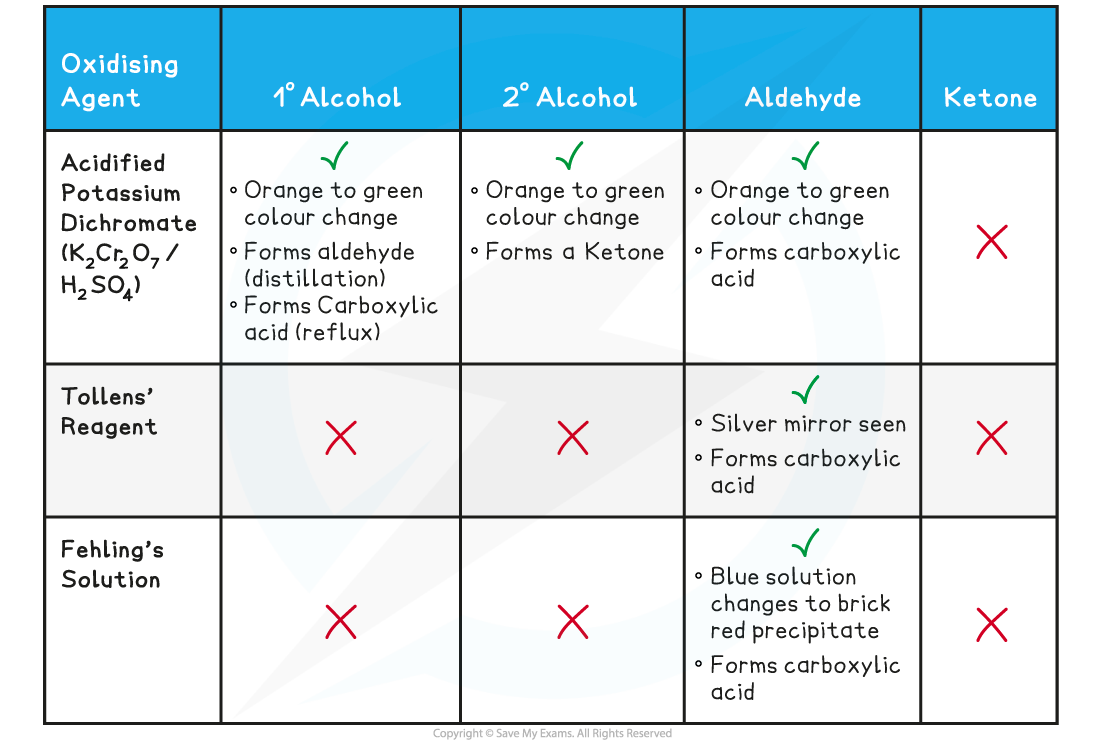
Summary of the Oxidation Reactions Table
转载自savemyexams

最新发布
© 2025. All Rights Reserved. 沪ICP备2023009024号-1









