- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
AQA A Level Chemistry复习笔记6.2.1 General Properties of Transition Metals
General Properties of Transition Metals
- Transition metals are elements with an incomplete d-subshell that can form at least one stable ion with an incomplete d-subshell
- This definition distinguishes them from d-block elements, because scandium and zinc do not fit the definition
- Scandium only forms the ion Sc3+, configuration [Ar] 3d0
- Zinc only forms the ion Zn2+, configuration [Ar] 3d10
- The elements of the first transition series are therefore titanium to copper
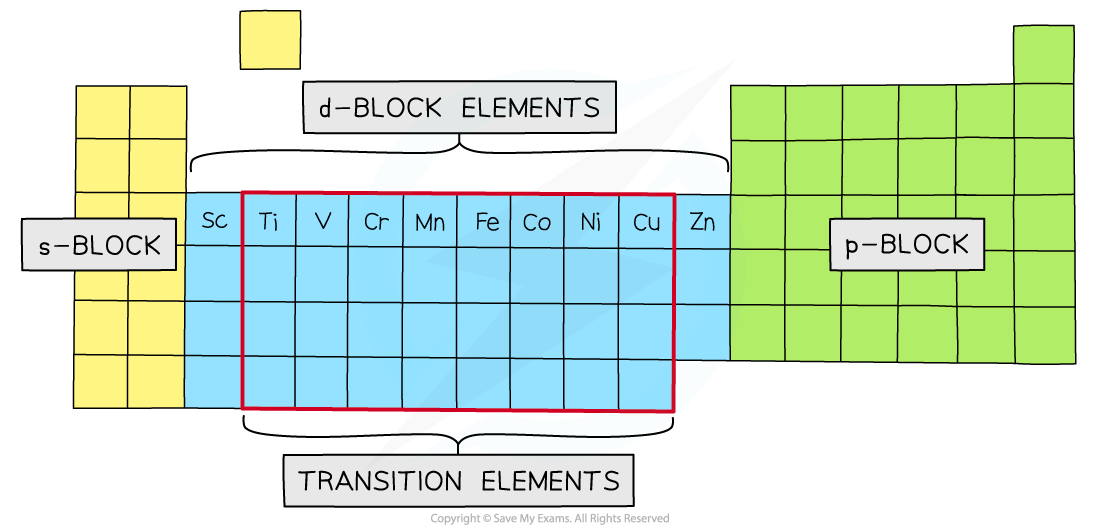
The transition elements and the d-block elements
Electron Configuration
- The full electronic configuration of the first d-series transition metals is shown in the table below
- Following the Aufbau Principle electrons occupy the lowest energy subshells first
- The 4s overlaps with the 3d subshell so the 4s is filled first
- Remember that you can abbreviate the first five subshells, 1s-3p, as [Ar] representing the configuration of argon( known as the argon core)
Table showing the electronic configuration of the first d-series transition elements
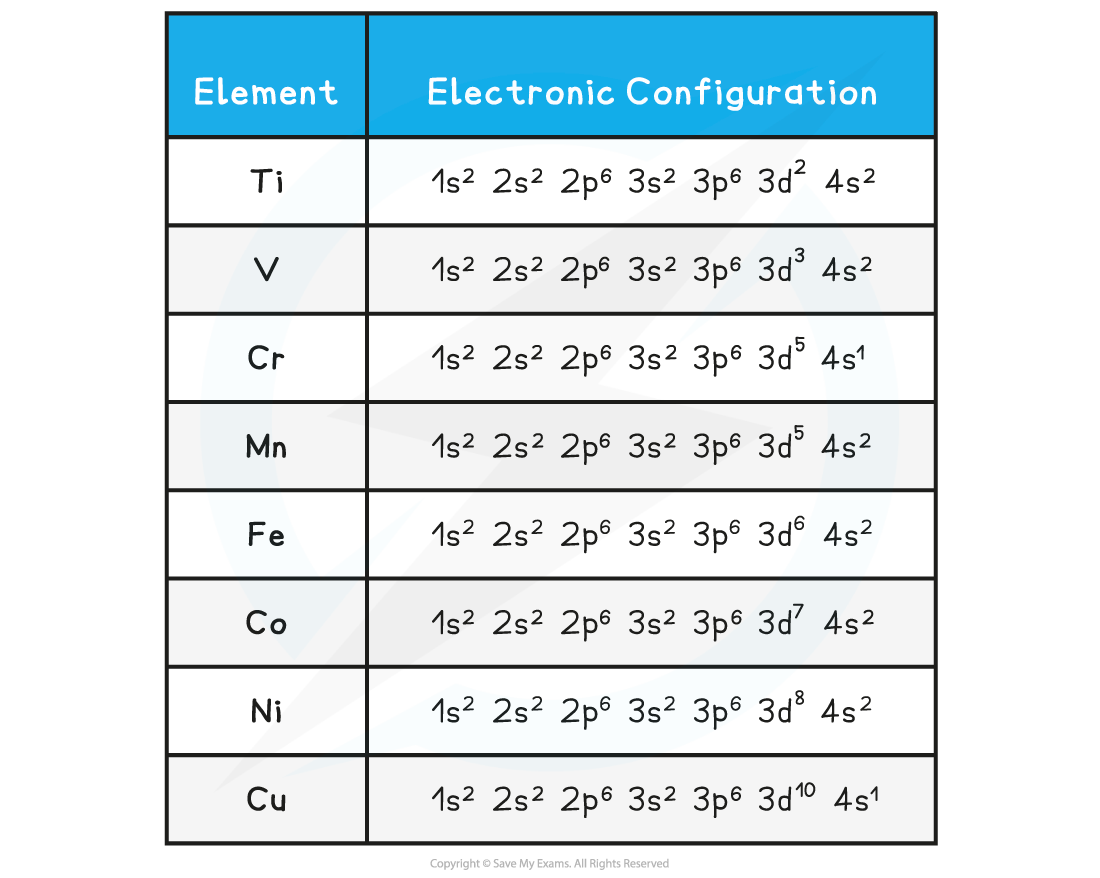
- From AS Chemistry you should recall two exceptions to the Aufbau Principle, chromium and copper
- In both cases an electron is promoted from the 4s to the 3d to achieve a half full and full d-subshell, respectively
- Chromium and copper have the following electron configurations, which are different to what you may expect:
- Cr is [Ar] 3d5 4s1 not [Ar] 3d4 4s2
- Cu is [Ar] 3d10 4s1 not [Ar] 3d9 4s2
- This is because the [Ar] 3d5 4s1 and [Ar] 3d10 4s1 configurations are energetically more stable
Worked Example
Writing electronic configuration of transition element ionsState the full electronic configuration of the manganese(III) ion
Answer
Step 1: Write out the electron configuration of the atom first:
Mn atomic number = 25
1s22s22p63s23p64s23d5
2 + 2 + 6 + 2 + 6 + 2 + 5 = 25 electrons
Step 2: Subtract the appropriate number of electrons starting from the 4s subshell
Mn(III) = 22 electrons
1s22s22p63s23p63d4
General properties
- Although the transition elements are metals, they have some properties unlike those of other metals on the periodic table, such as:
- Variable oxidation states
- Form complex ions
- Form coloured compounds
- Behave as catalysts
Variable Oxidation States
- Like other metals on the periodic table, the transition elements will lose electrons to form positively charged ions
- However, unlike other metals, transition elements can form more than one positive ion
- They are said to have variable oxidation states
- Because of this, Roman numerals are used to indicate the oxidation state on the metal ion
- For example, the metal sodium (Na) will only form Na+ ions (no Roman numerals are needed, as the ion formed by Na will always have an oxidation state of +1)
- The transition metal iron (Fe) can form Fe2+ (Fe(II)) and Fe3+ (Fe(III)) ions
Forming Complex ions
- Another property of transition elements caused by their ability to form variable oxidation states, is their ability to form complex ions
- A complex ion is a molecule or ion, consisting of a central metal atom or ion, with a number of molecules or ions surrounding it
- A molecule or ion surrounding the central metal atom or ion is called a ligand
- Due to the different oxidation states of the central metal ions, a different number and wide variety of ligands can form bonds with the transition element
- For example, the chromium(III) ion can form [Cr(NH3)6]3+, [Cr(OH)6]3- and [Cr(H2O)6]3+ complex ions
Forming coloured compounds
- Another characteristic property of transition elements is that their compounds are often coloured
- For example, the colour of the [Cr(OH)6]3- complex (where oxidation state of Cr is +3) is dark green
- Whereas the colour of the [Cr(NH3)6]3+ complex (oxidation state of Cr is still +3) is purple
Transition elements as catalysts
- Since transition elements can have variable oxidation states, they make excellent catalysts
- During catalysis, the transition element can change to various oxidation states by gaining electrons or donating electrons from reagents within the reaction
- Substances can also be adsorbed onto their surface and activated in the process
Complex Ions
- Transition element ions can form complexes which consist of a central metal ion and ligands
- A ligand is a molecule or ion that forms a co-ordinate bond with a transition metal by donating a pair of electrons to the bond
- This is the definition of a Lewis base - electron pair donor
- This means ligands have a negative charge or a lone pair of electrons capable of being donated
- This definition may seem familiar: a ligand is the same as a nucleophile
- Different ligands can form different numbers of dative bonds to the central metal ion in a complex
- Some ligands can form one dative bond to the central metal ion
- Other ligands can form two dative bonds, and some can form multiple dative bonds
- Co-ordination number is number of co-ordinate bonds to the central metal atom or ion
Examples of ligands Table
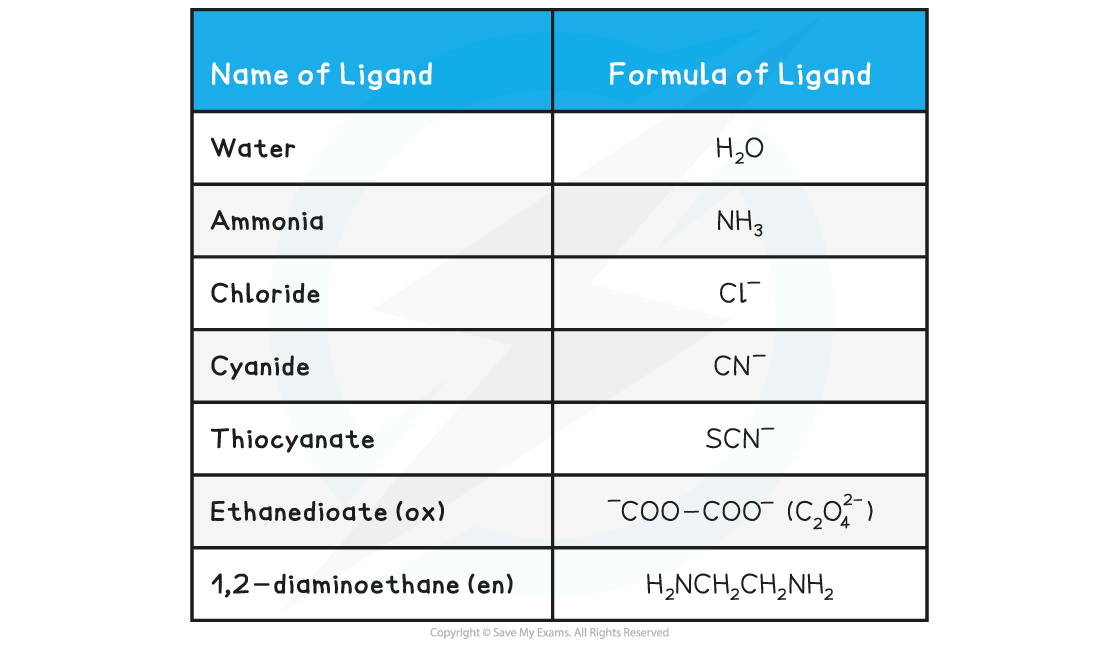
Monodentate Ligands
- Monodentate ligands can form only one dative bond to the central metal ion
- Examples of monodentate ligands are:
- Water (H2O) molecules
- Ammonia (NH3) molecules
- Chloride (Cl–) ions
- Cyanide (CN–) ions
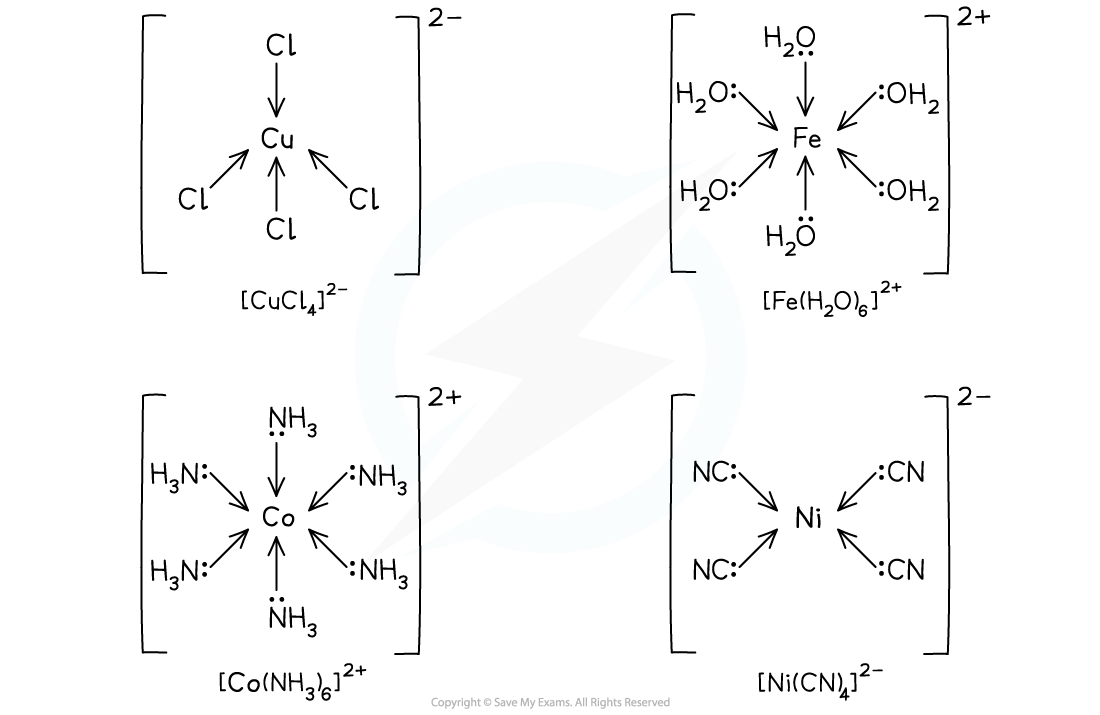
Examples of complexes with monodentate ligands
Bidentate Ligands
- Bidentate ligands can each form two dative bonds to the central metal ion
- This is because each ligand contains two atoms with lone pairs of electrons
- Examples of bidentate ligands are:
- 1,2-diaminoethane (H2NCH2CH2NH2) which is also written as ‘en’
- Ethanedioate ion (C2O42- ) which is sometimes written as ‘ox’
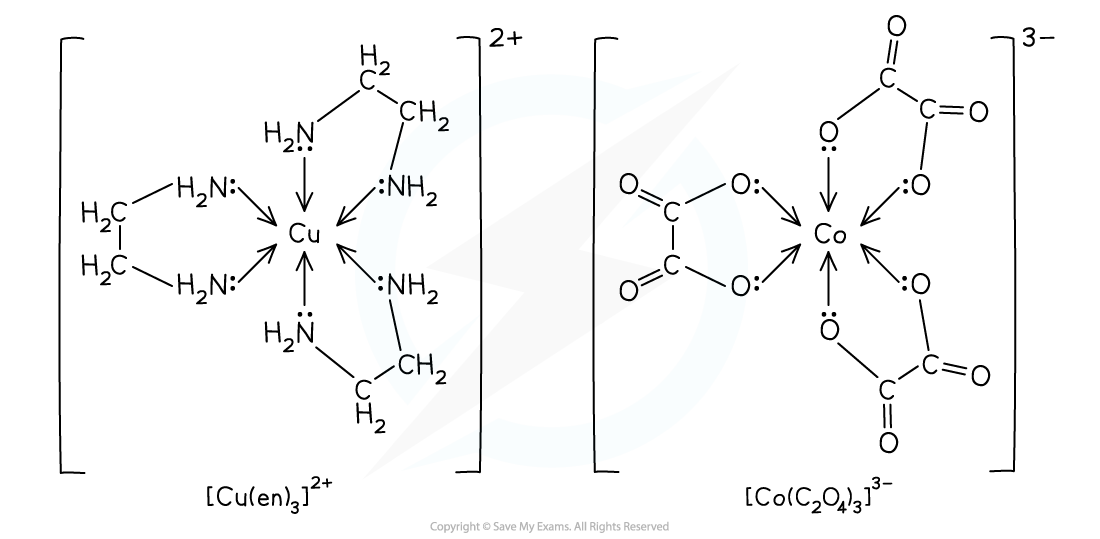
Examples of complexes with bidentate ligands
Multidentate Ligands
- Some ligands contain more than two atoms with lone pairs of electrons
- These ligands can form more than two dative bonds to the and are said to be multidentate ligands
- An example of a multidentate ligand is EDTA4-, which is a hexadentate ligand as it forms 6 dative covalent bonds to the central metal ion
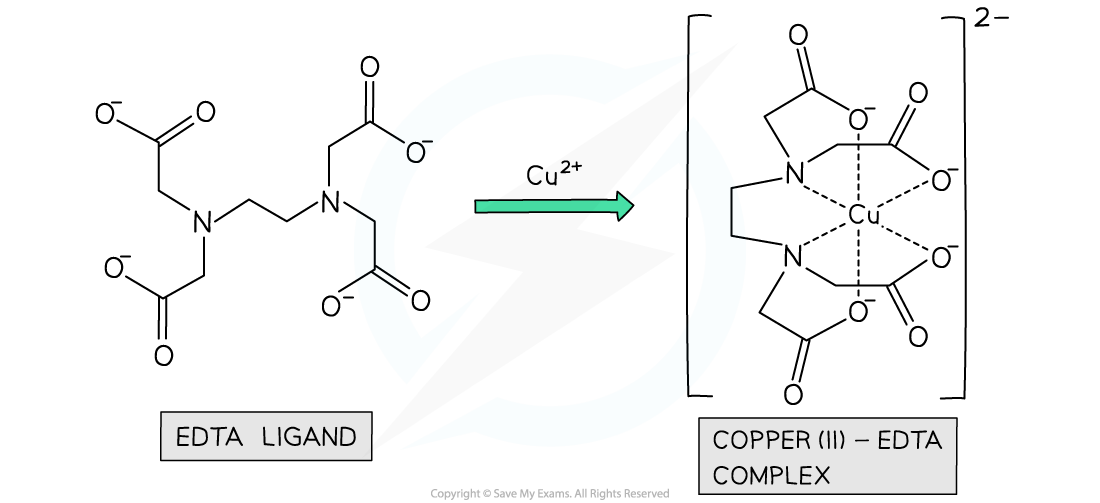
Example of a polydentate ligand complex
Complexes with water & ammonia molecules
- Water and ammonia molecules are examples of neutral ligands
- Both ligands contain a lone pair of electrons which can be used to form a dative covalent bond with the central metal ion
- In water, this is the lone pair on the oxygen atom
- In ammonia, it is the lone pair on the nitrogen atom
- Since water and ammonia are small ligands, 6 of them can usually fit around a central metal ion, each donating a lone pair of electrons, forming 6 dative bonds
- Since there are 6 dative bonds, the coordination number for the complex is 6
- The overall charge of a complex is the sum of the charge on the central metal ion, and the charges on each of the ligands
- A complex with cobalt(II) or chromium(II) as a central metal ion, and water or ammonia molecules as ligands, will have an overall charge of 2+
- The central metal ion has a 2+ charge and the ligands are neutral
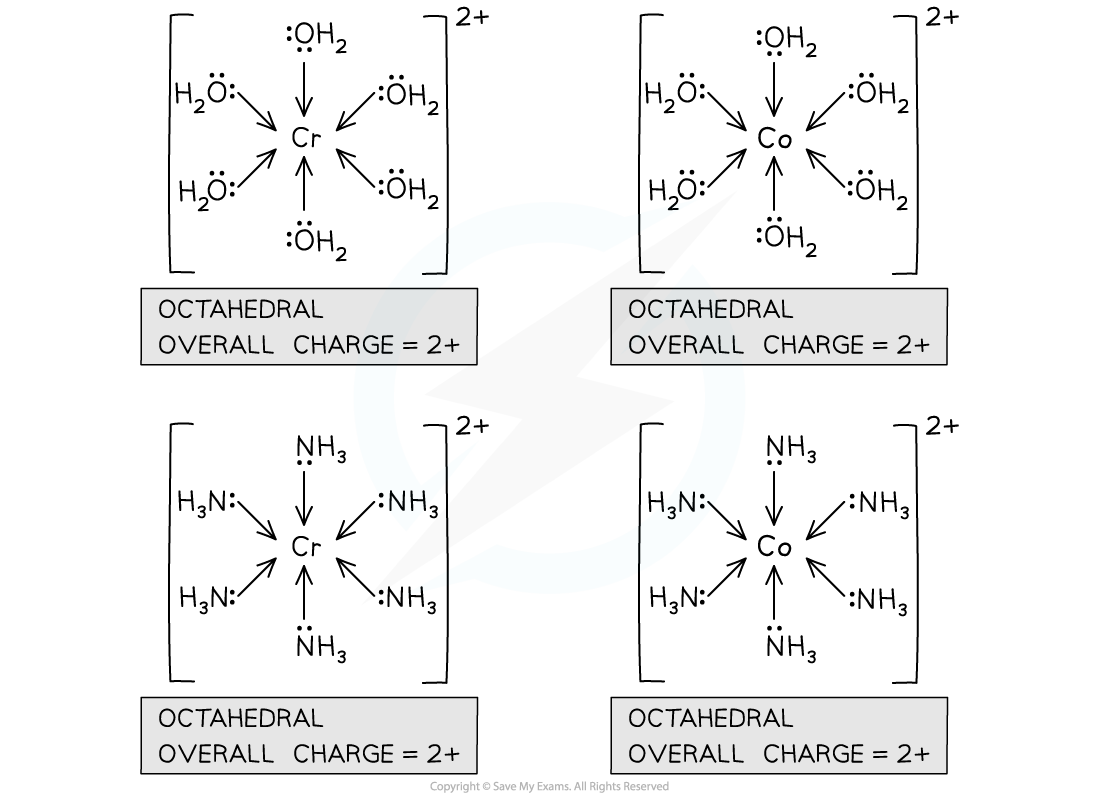
Cobalt(II) and chromium(II) form octahedral complexes with ammonia and water ligands
Complexes with hydroxide & chloride ions
- Hydroxide and chloride ions are examples of negatively charged ligands
- Both ligands contain a lone pair of electrons which can be used to form a dative covalent bond with the central metal ion
- Hydroxide ligands are small, so 6 of them can fit around a central metal ion and the complex formed will have a coordination number of 6
- Chloride ligands are large ligands, so only 4 of them will fit around a central metal ion
- Complexes with 4 chloride ligands will have a coordination number of 4
- A complex with cobalt(II) or copper(II) as a central metal ion and chloride ions as ligands, will have an overall charge of 2-
-
- The central metal ion has a charge of 2+
- Each chloride ligand has a charge of 1-
- There are 4 chloride ligands in the complex, so the overall negative charge is 4-
- The overall positive charge is 2+
- Therefore, the overall charge of the complex is 2-
-
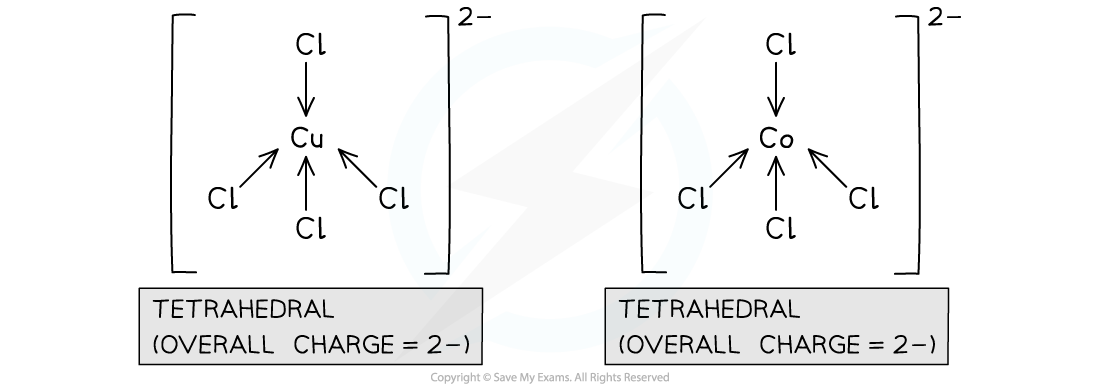
Cobalt(II) and copper(II) form tetrahedral complexes with chloride ligands
- A complex with chromium(III) as a central metal ion and hydroxide ions as ligands, will have an overall charge of 3-
- The central metal ion has a charge of 3+
- Each hydroxide ligand has a charge of 1-
- There are 6 hydroxide ligands in the complex, so the overall negative charge is 6-
- The overall positive charge is 3+
- Therefore, the overall charge on the complex is -3
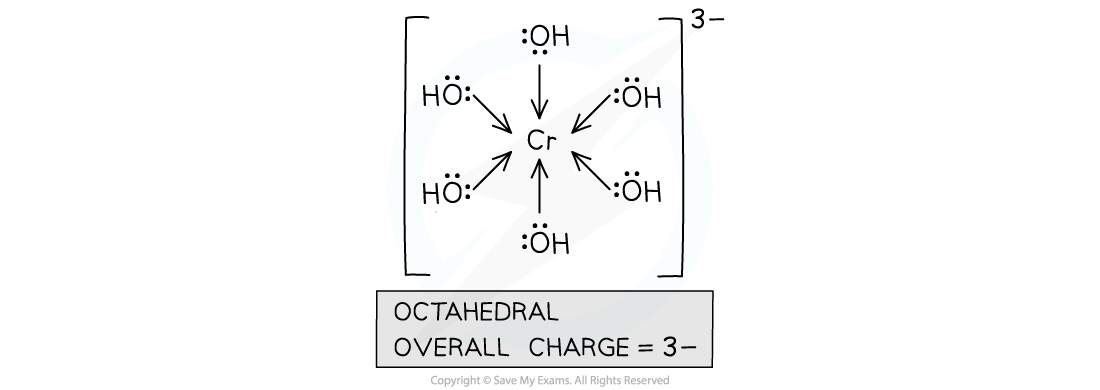
Chromium(III) ions form a complex ion with hydroxide ions
转载自savemyexams

最新发布
© 2025. All Rights Reserved. 沪ICP备2023009024号-1









