- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
AQA A Level Chemistry复习笔记5.4.1 Representing Cells
Representing Cells
- Electrochemical cells generate electricity from spontaneous redox reactions
- For example:
Zn (s) + CuSO4 (aq)→ Cu (s) + ZnSO4 (aq)
-
- Instead of electrons being transferred directly from the zinc to the copper ions, a cell is built which separates the two redox processes
- For example:
Zn (s) ⇌ Zn2+ (aq) + 2e–
- If a rod of metal is dipped into a solution of its own ions, an equilibrium is set up
- Each part of the cell is called a half cell
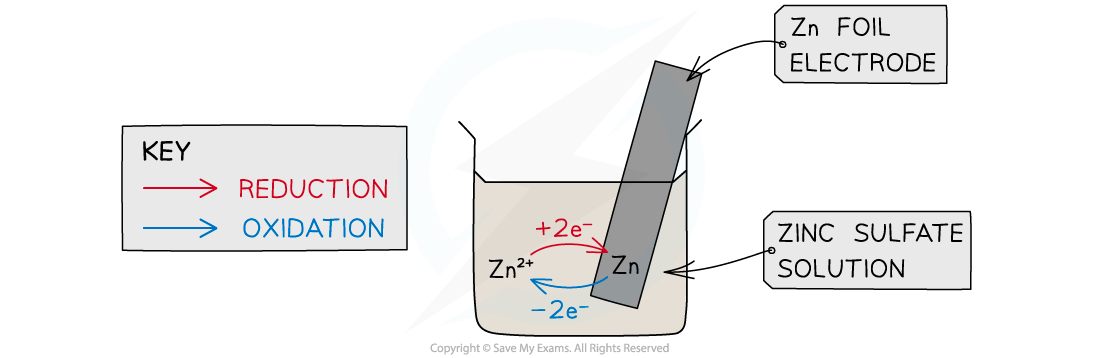
When a metal is dipped into a solution containing its ions an equilibrium is established between the metal and its ions. This is the basis of a half cell in an electrochemical cell.
- This is a half cell and the strip of metal is an electrode
- The position of the equilibrium determines the potential difference between the metal strip and the solution of metal
- The Zn atoms on the rod can deposit two electrons on the rod and move into solution as Zn2+ ions:
Zn(s) ⇌ Zn2+(aq) + 2e–
- This process would result in an accumulation of negative charge on the zinc rod
- Alternatively, the Zn2+ ions in solution could accept two electrons from the rod and move onto the rod to become Zn atoms:
Zn2+(aq) + 2e– ⇌ Zn(s)
- This process would result in an accumulation of positive charge on the zinc rod
- In both cases, a potential difference is set up between the rod and the solution
- This is known as an electrode potential
- A similar electrode potential is set up if a copper rod is immersed in a solution containing copper ions (e.g. CuSO4), due to the following processes:
Cu2+(aq) + 2e– ⇌ Cu(s) – reduction (rod becomes positive)
Cu(s) ⇌ Cu2+(aq) + 2e– – oxidation (rod becomes negative)
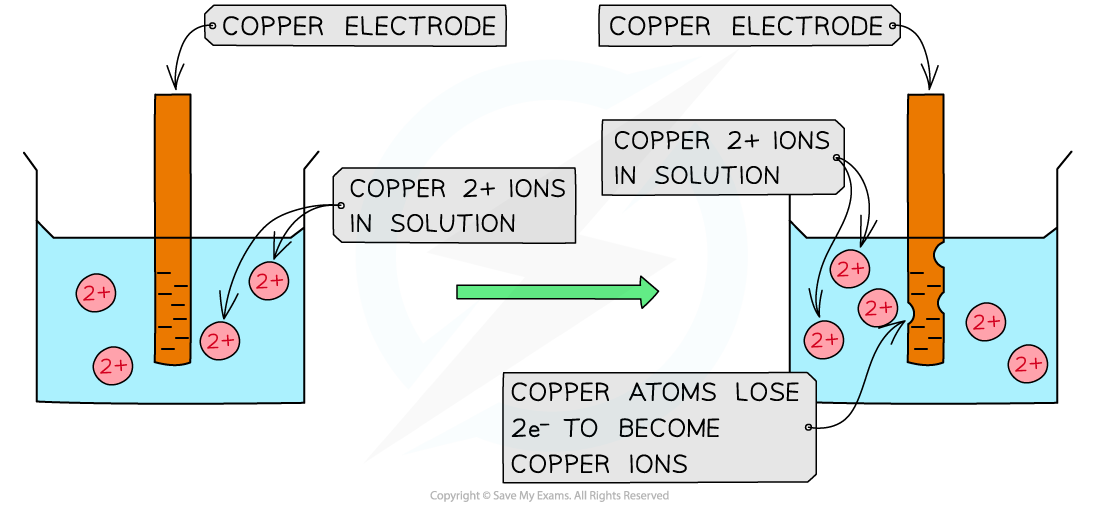
Oxidation of copper atoms
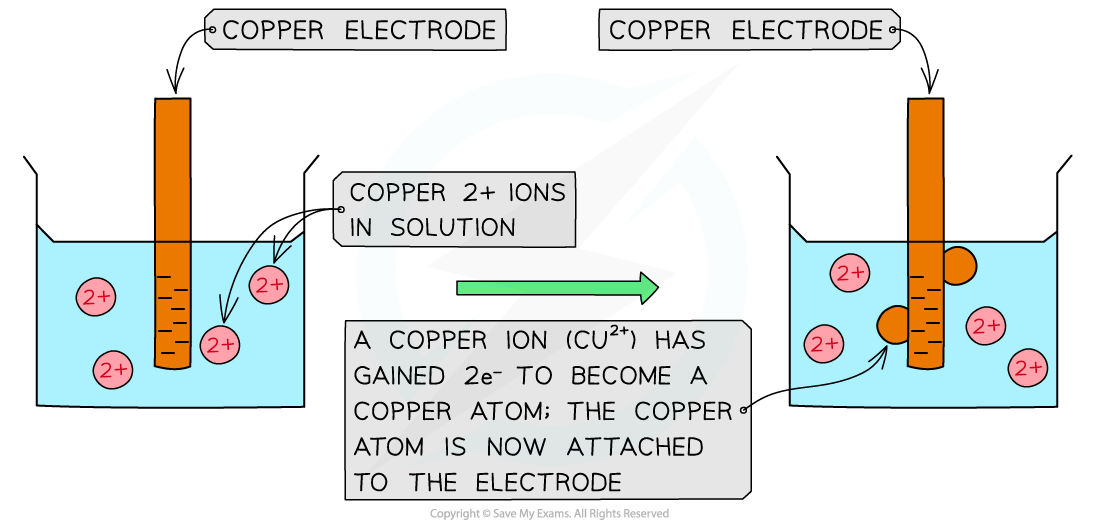
Reduction of copper(II) ions
- Note that a chemical reaction is not taking place – there is simply a potential difference between the rod and the solution
- The potential difference will depend on
- the nature of the ions in solution
- the concentration of the ions in solution
- the type of electrode used
- the temperature
Electrode potential
- The electrode (reduction) potential (E) is a value which shows how easily a substance is reduced
- These are demonstrated using reversible half equations
- This is because there is a redox equilibrium between two related species that are in different oxidation states
- When writing half equations for this topic, the electrons will always be written on the left-hand side (demonstrating reduction)
- The position of equilibrium is different for different species, which is why different species will have different electrode (reduction) potentials
- The more positive (or less negative) an electrode potential, the more likely it is for that species to undergo reduction
- The equilibrium position lies more to the right
- For example, the positive electrode potential of bromine below, suggests that it is likely to get reduced and form bromide (Br-) ions
Br2(l) + 2e- ⇌ 2Br-(aq) E = +1.09 V
- The more negative (or less positive) the electrode potential, the less likely it is that reduction of that species will occur
- The equilibrium position lies more to the left
- For example, the negative electrode potential of sodium suggests that it is unlikely that the sodium (Na+) ions will be reduced to sodium (Na) atoms
Na+(aq) + e- ⇌ Na(s) E = -2.71 V
Conventional Representation of Cells
- Chemists use a type of shorthand convention to represent electrochemical cells
- In this convention:
- A solid vertical (or slanted) line shows a phase boundary, that is an interface between a solid and a solution
- A double vertical line (sometimes shown as dashed vertical lines) represents a salt bridge
- A salt bridge has mobile ions that complete the circuit
- Potassium chloride and potassium nitrate are commonly used to make the salt bridge as chlorides and nitrates are usually soluble
- This should ensure that no precipitates form which can affect the equilibrium position of the half cells
- The substance with the highest oxidation state in each half cell is drawn next to the salt bridge
- The cell potential difference is shown with the polarity of the right hand electrode
- The cell convention for the zinc and copper cell would be
Zn (s)∣Zn2+ (aq) ∥Cu2+ (aq)∣Cu (s) E cell = +1.10 V
- This tells us the copper half cell is more positive than the zinc half cell, so that electrons would flow from the zinc to the copper
- The same cell can be written as:
Cu (s)∣Cu2+ (aq) ∥Zn2+ (aq)∣Zn (s) E cell = -1.10 V
- The polarity of the right hand half cell is negative, so we can still tell that electrons flow from the zinc to the copper half cell
Worked Example
Writing a cell diagram
If you connect an aluminium electrode to a zinc electrode, the voltmeter reads 0.94V and the aluminium is the negative. Write the conventional cell diagram to the reaction.
Answer
Al (s)∣Al3+ (aq) ∥ Zn2+ (aq)∣Zn (s) E cell = +0.94 V
It is also acceptable to include phase boundaries on the outside of cells as well:
∣ Al (s)∣Al3+ (aq) ∥ Zn2+ (aq)∣Zn (s) ∣ E cell = +0.94 V
Exam Tip
Students often confuse the redox processes that take place in electrochemical cells.
- Oxidation takes place at the negative electrode.
- Reduction takes place at the positive electrode.
Remember, oxidation is the loss of electrons, so you are losing electrons at the negative.
∣ Al (s)∣Al3+ (aq) ∥Zn2+ (aq)∣Zn (s) ∣ Ecell = +0.94 V
转载自savemyexams

最新发布
© 2025. All Rights Reserved. 沪ICP备2023009024号-1









