- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
AQA A Level Chemistry复习笔记5.3.1 Kp Expressions
Kp Expressions
- We have seen previously that equilibrium reactions can be quantified by reference to an equilibrium expression and equilibrium constant
- The equilibrium expression links the equilibrium constant, Kc, to the concentrations of reactants and products at equilibrium taking the stoichiometry of the equation into account
- So, for a given reaction:
aA + bB ⇌ cC + dD
Kc is defined as follows:
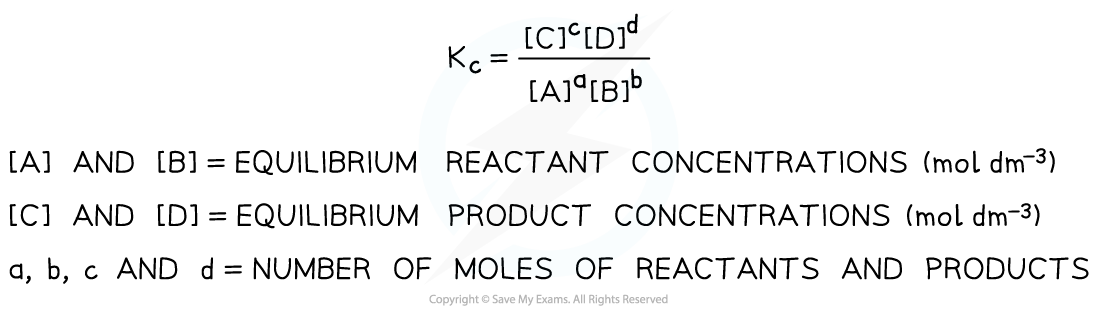
Equilibrium expression linking the equilibrium concentration of reactants and products at equilibrium
- Solids are ignored in equilibrium expressions
- The Kc of a reaction is constant and only changes if the temperature of the reaction changes
Gaseous Equilibria
- This section covers how we manage gases in calculating the equilibrium constant and how an equilibrium yield is affected by the partial pressures of reactants and products
- Many industrial process involve reactions between gases so this application has important consequences for controlling reaction conditions
- In the generic example above, if all the substances are gases, we can show the equation with that state symbol
aA (g) + bB (g) ⇌ cC (g) + dD (g)
- We can write a different equilibrium expression in terms of the partial pressure of the gases
- This equilibrium constant is called Kp and is defined as follows
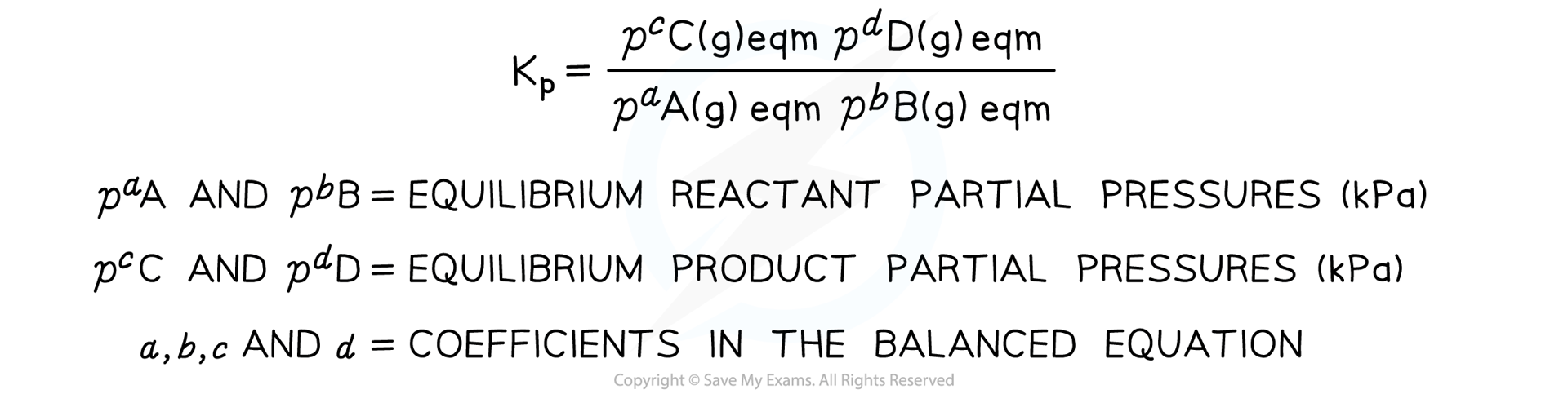
Equilibrium expression linking the partial pressures of reactants and products at equilibrium
- Solids and liquids are ignored in Kp equilibrium expressions
- The Kp of a reaction is constant and only changes if the temperature of the reaction changes
Worked Example
Write a Kp expression for the following equilibria and deduce the units of Kp :
- N2 (g) + 3H2 (g) ⇌ 2NH3 (g)
- 2SO2 (g) + O2 (g) ⇌ 2SO3 (g)
Answer 1

Answer 2

转载自savemyexams

最新发布
© 2026. All Rights Reserved. 沪ICP备2023009024号-1









