- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
AQA A Level Chemistry复习笔记2.3.1 Physical Properties of Group 7
Group 7: Physical Properties
- The group 7 elements are called halogens
- The halogens have uses in water purification and as bleaching agents (chlorine), as flame-retardants and fire extinguishers (bromine) and as antiseptic and disinfectant agents (iodine)
Colours
- All halogens have distinct colours which get darker going down the group
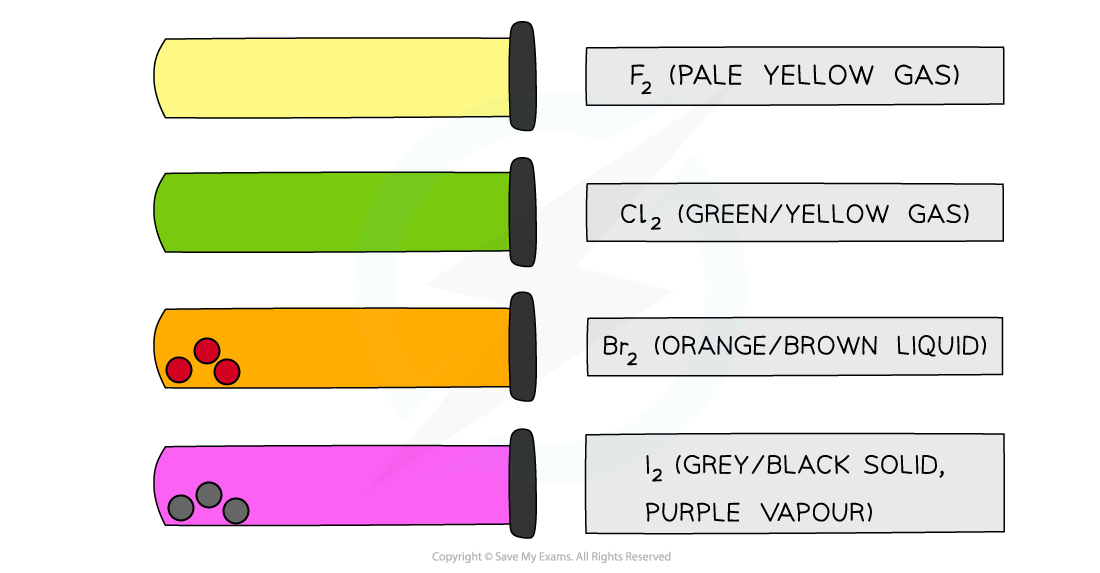
The colours of the Group 7 elements get darker going down the group
Volatility
- Volatility refers to how easily a substance can evaporate
- A volatile substance will have a low boiling point
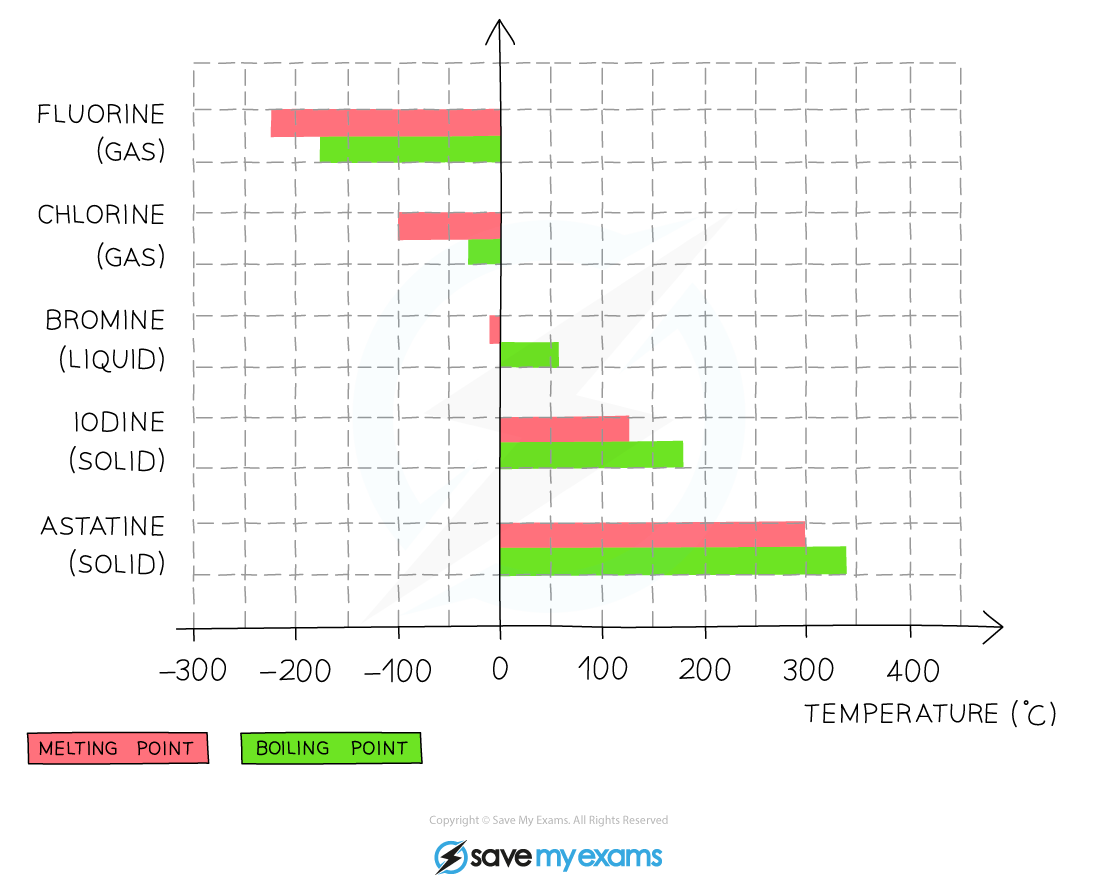
The melting and boiling points of the Group 7 elements increase going down the group which indicates that the elements become less volatile
- Going down the group, the boiling point of the elements increases which means that the volatility of the halogens decreases
- This means that fluorine is the most volatile and iodine the least volatile
Bond Strength
- Halogens are diatomic molecules in which covalent bonds are formed by overlapping their orbitals
- In a covalent bond, the bonding pair of electrons is attracted to the nuclei on either side and it is this attraction that holds the molecule together
- Going down the group, the atomic size of the halogens increases
- The bonding pair of electrons get further away from the halogen nucleus and are therefore less strongly attracted towards it
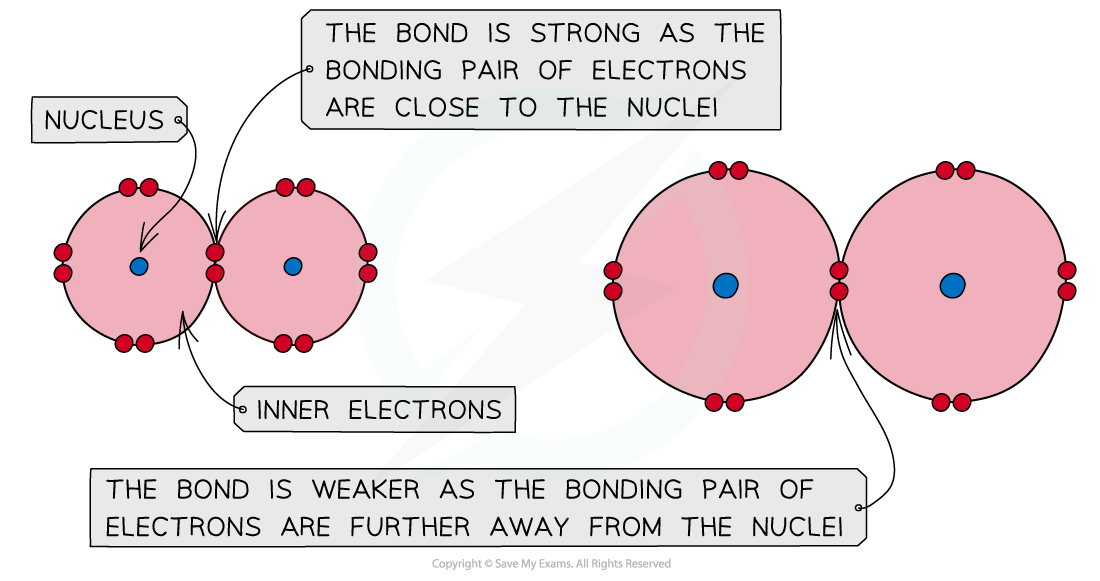
A covalent bond is formed by the orbital overlap of two atoms and the attraction of electrons towards the nuclei; the bigger the atom, the weaker the covalent bond
Bond enthalpy data
- The bond strength of the halogen molecules decreases going down the group
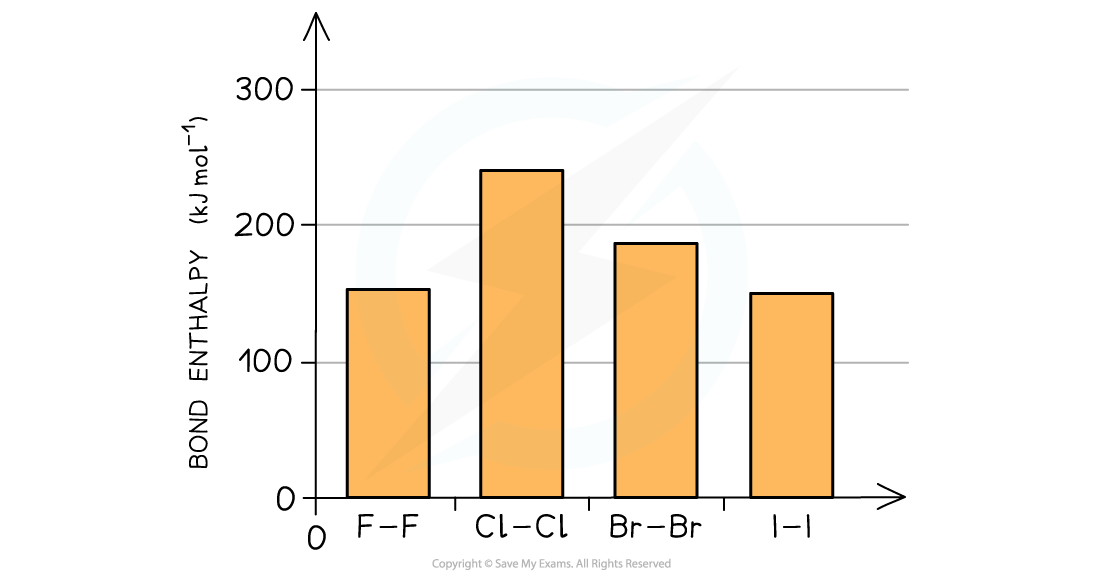
The bond enthalpies decrease indicating that the bond strengths decrease going down the group
- Bond enthalpy is the energy needed to break one mole of covalent bonds
- The higher the bond enthalpy, the stronger the bond
- An exception to this is fluorine which has a smaller bond enthalpy than chlorine and bromine
- Fluorine is so small that when two atoms of fluorine get together their lone pairs get so close that they cause significant repulsion counteracting the attraction between the bonding pair of electrons and two nuclei

The lone pairs on fluorine get so close to each other in a fluorine molecule that they cause repulsion which decreases the bond strength
Dipole Forces & Volatility
- Halogens are non-metals and are diatomic molecules at room temperature
- This means that they exist as molecules which are made up of two similar atoms, such as F2
- The halogens are simple molecular structures with weak van der Waals’ forces between the diatomic molecules caused by instantaneous dipole-induced dipole forces
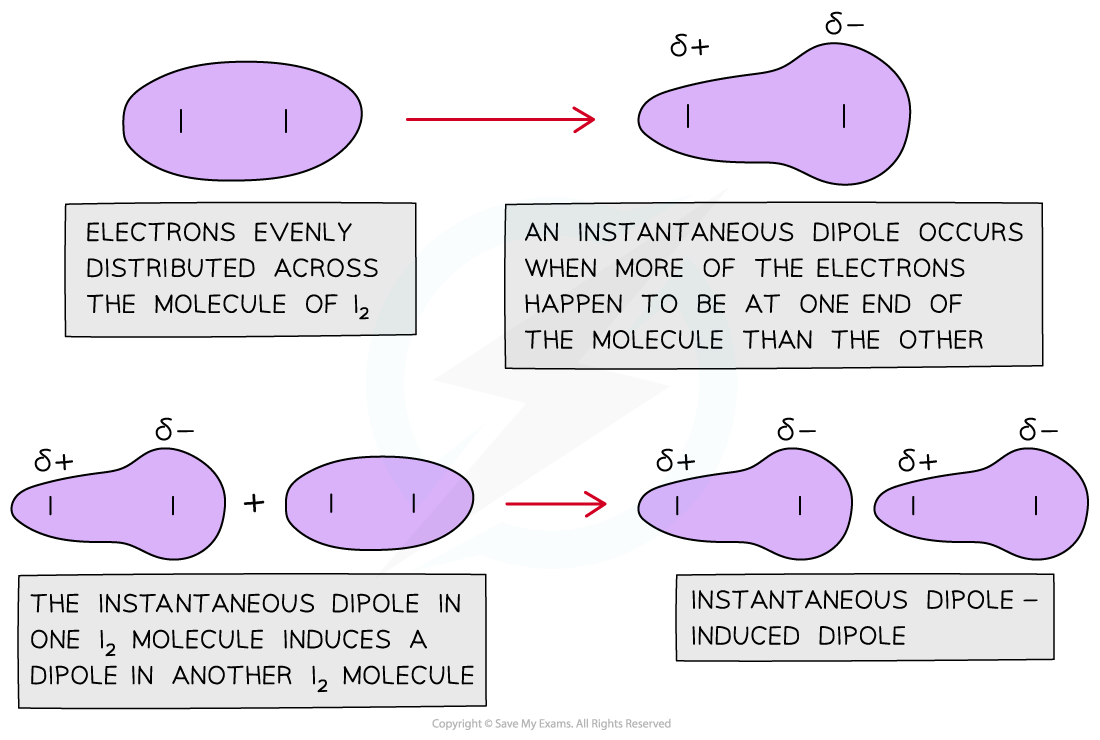
The diagram shows that a sudden imbalance of electrons in a nonpolar molecule can cause an instantaneous dipole. When this molecule gets close to another non-polar molecule it can induce a dipole as the cloud of electrons repel the electrons in the neighbouring molecule to the other side
- The more electrons there are in a molecule, the greater the instantaneous dipole-induced dipole forces
- Therefore, the larger the molecule the stronger the van der Waals’ forces between molecules
- This is why as you go down the group, it gets more difficult to separate the molecules and the melting and boiling points increase
- As it gets more difficult to separate the molecules, the volatility of the halogens decreases going down the group
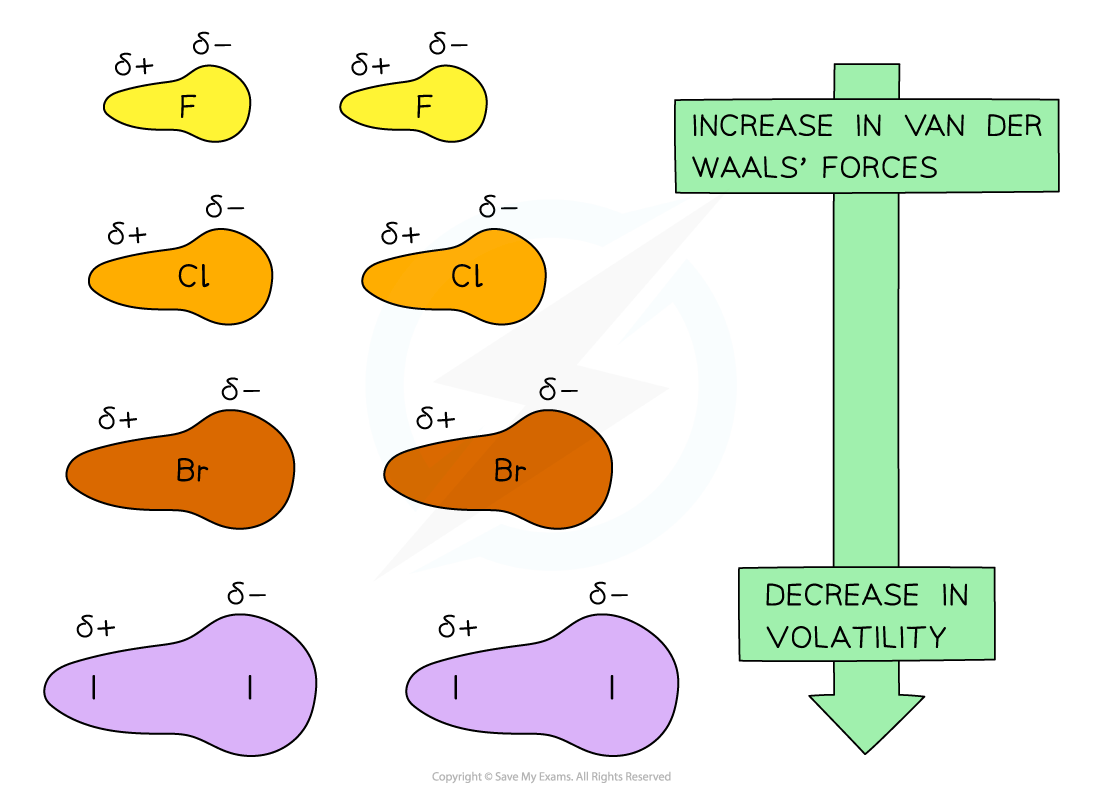
Going down the group, the van der Waals’ forces increase due to an increased number of electrons in the molecules which means that the volatility decreases
转载自savemyexams

最新发布
© 2025. All Rights Reserved. 沪ICP备2023009024号-1









