- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
AQA A Level Chemistry复习笔记1.1.1 Fundamental Particles
Structure of an Atom
- All matter is composed of atoms, which are the smallest parts of an element that can take place in chemical reactions
- Atoms are mostly made up of empty space around a very small, dense nucleus that contains protons and neutrons
- The nucleus has an overall positive charge
- The protons have a positive charge and the neutrons have a neutral charge
- Negatively charged electrons are found in orbitals in the empty space around the nucleus
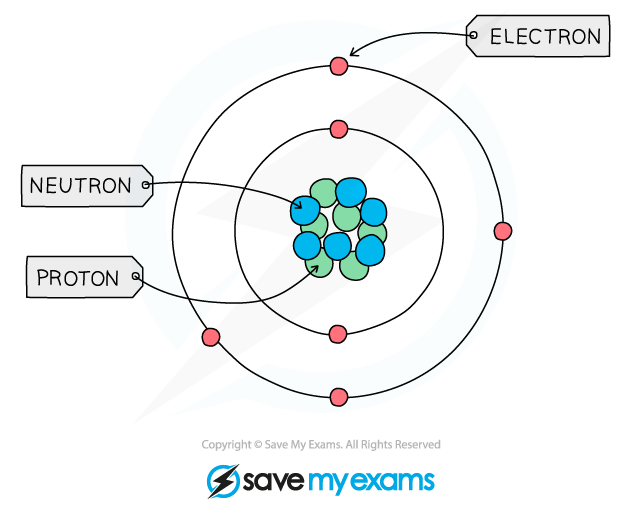
The basic structure of an atom (not to scale)
Subatomic Particles
- Subatomic particles are the particles an element is made up of and include protons, neutrons and electrons
- These subatomic particles are so small that it is not possible to measure their masses and charges using conventional units (such as grams and coulombs)
- Instead, their masses and charges are compared to each other using ‘relative atomic masses’ and ‘relative atomic charges’
- These are not actual charges and masses but they are charges and masses of particles relative to each other
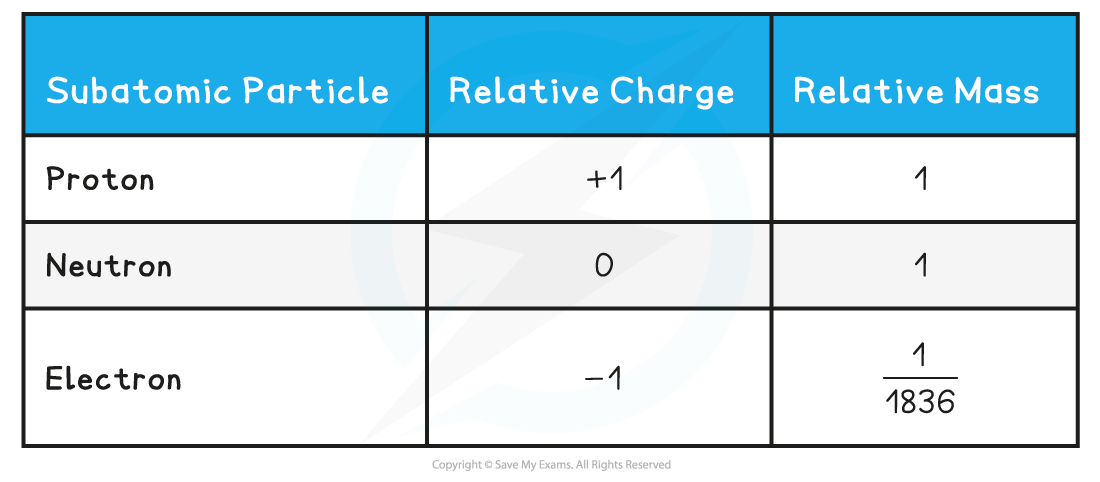
- Protons and neutrons have a very similar mass so each is assigned a relative mass of 1 whereas electrons are 1836 times smaller than a proton and neutron
- Protons are positively charged, electrons negatively charged and neutrons are neutral
- The relative mass and charge of the subatomic particles are:
Relative mass & charge of subatomic particles table
Exam Tip
The relative mass of an electron is almost negligible.The charge of a single electron is -1.602 x 10-19 coulombs whereas the charge of a proton is +1.602 x 10-19 coulombs, however, relative to each other, their charges are -1 and +1 respectively.
Atoms: Key Terms
- The atomic number (or proton number) is the number of protons in the nucleus of an atom and has symbol Z
- The atomic number is equal to the number of electrons present in a neutral atom of an element
- Eg. the atomic number of lithium is 3 which indicates that the neutral lithium atom has 3 protons and 3 electrons
- The mass number (or nucleon number) is the total number of protons and neutrons in the nucleus of an atom and has symbol A
- The number of neutrons can be calculated by:
Number of neutrons = mass number - atomic number
-
- Protons and neutrons are also called nucleons
Exam Tip
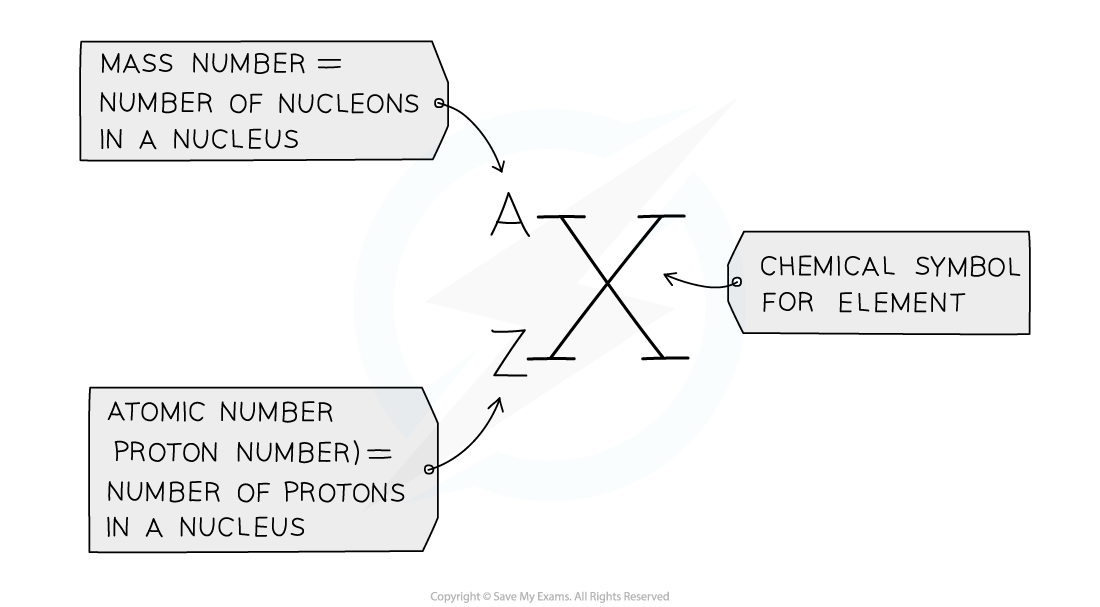
The mass (nucleon) and atomic (proton) number are given for each element in the Periodic Table
转载自savemyexams

早鸟钜惠!翰林2025暑期班课上线

最新发布
© 2025. All Rights Reserved. 沪ICP备2023009024号-1








