- Enzymes have a specific optimum temperature – the temperature at which they catalyse a reaction at the maximum rate
- Lower temperatures either prevent reactions from proceeding or slow them down:
- Molecules move relatively slow
- Lower frequency of successful collisions between substrate molecules and active site of enzyme
- Less frequent enzyme-substrate complex formation occurs
- Substrate and enzyme collide with less energy, making it less likely for bonds to be formed or broken (stopping the reaction from occurring)
- Higher temperatures speed up reactions:
- Molecules move more quickly
- Higher frequency successful collisions between substrate molecules and active site of enzyme
- More frequent enzyme-substrate complex formation
- Substrate and enzyme collide with more energy, making it more likely for bonds to be formed or broken (allowing the reaction to occur)
- However, as temperatures continue to increase, the rate at which an enzyme catalyses a reaction drops sharply, as the enzyme begins to denature:
- Bonds (eg. hydrogen bonds) holding the enzyme molecule in its precise shape start to break
- This causes the tertiary structure of the protein (ie. the enzyme) to change
- This permanently damages the active site, preventing the substrate from binding
- Denaturation has occurred if the substrate can no longer bind
- Very few human enzymes can function at temperatures above 50°C
- This is because humans maintain a body temperature of about 37°C, therefore even temperatures exceeding 40°C will cause the denaturation of enzymes
- High temperatures cause the hydrogen bonds between amino acids to break, changing the conformation of the enzyme
- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
AQA A Level Biology复习笔记6.4.1 Principles of Homeostasis
Homeostasis
- In order to function properly and efficiently, organisms have different control systems that ensure their internal conditions are kept relatively constant
- Physiological control systems maintain the internal environment within restricted limits through a process known as homeostasis
- Homeostasis is critically important for organisms as it ensures the maintenance of optimal conditions for enzyme action and cell function
- Sensory cells can detect information about the conditions inside and outside of the body
- Examples of physiological factors that are controlled by homeostasis in mammals include:
- Core body temperature
- Metabolic waste (eg. carbon dioxide and urea)
- Blood pH
- Concentration of glucose in the blood
- Water potential of the blood
- Concentration of the respiratory gases (carbon dioxide and oxygen) in the blood
- Homeostatic mechanisms in mammals require information to be transferred between different parts of the body
- There are two coordination systems in mammals that do this:
- The nervous system
- The endocrine system
The nervous system
- The human nervous system consists of:
- The central nervous system (CNS) – the brain and the spinal cord
- The peripheral nervous system (PNS) – all of the nerves in the body
- It allows us to make sense of our surroundings and respond to them and coordinate and regulate body functions
- Information is sent through the nervous system as nerve impulses – electrical signals that pass along nerve cells known as neurones
- A bundle of neurones is known as a nerve
- Neurones coordinate the activities of sensory receptors (eg. those in the eye), decision-making centres in the central nervous system, and effectors such as muscles and glands
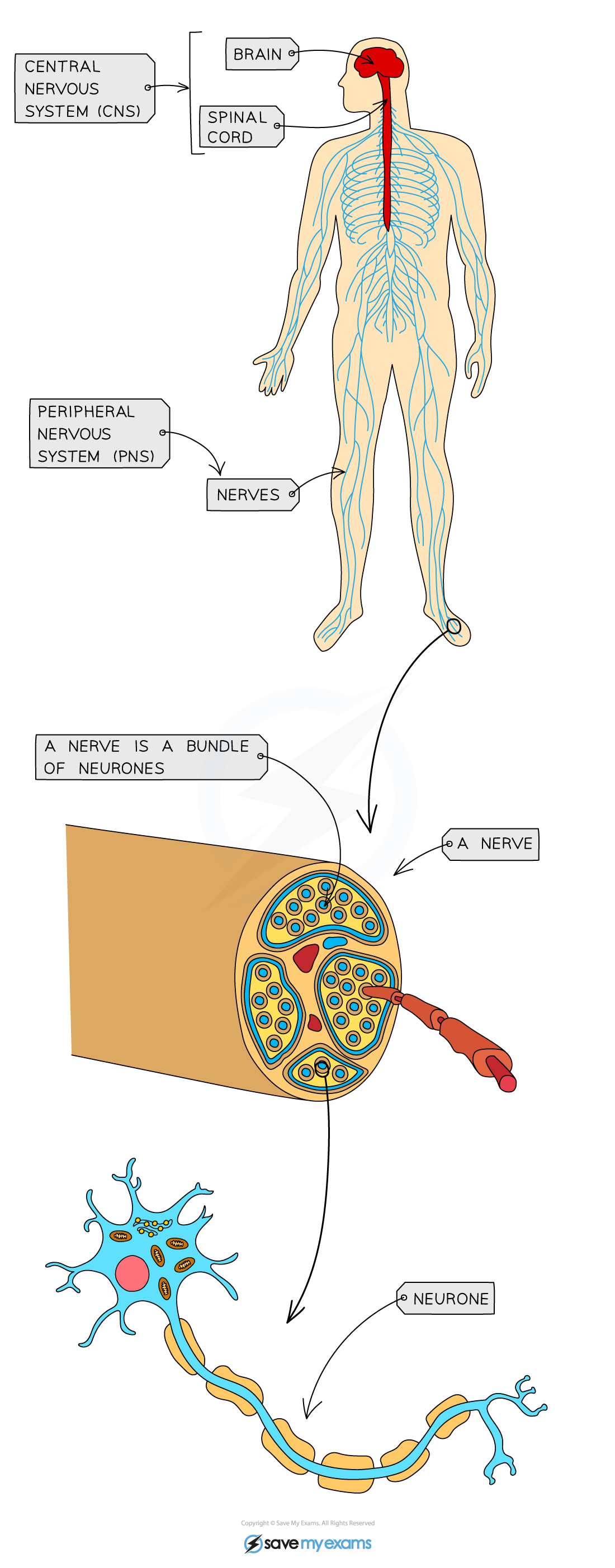
The human nervous system
The endocrine system
- A hormone is a chemical substance produced by an endocrine gland and carried by the blood
- They are chemicals which transmit information from one part of the organism to another and bring about a change
- They alter the activity of one or more specific target organs
- Hormones are used to control functions that do not need instant responses
- The endocrine glands that produce hormones in animals are known collectively as the endocrine system
- A gland is a group of cells that produces and releases one or more substances (a process known as secretion)
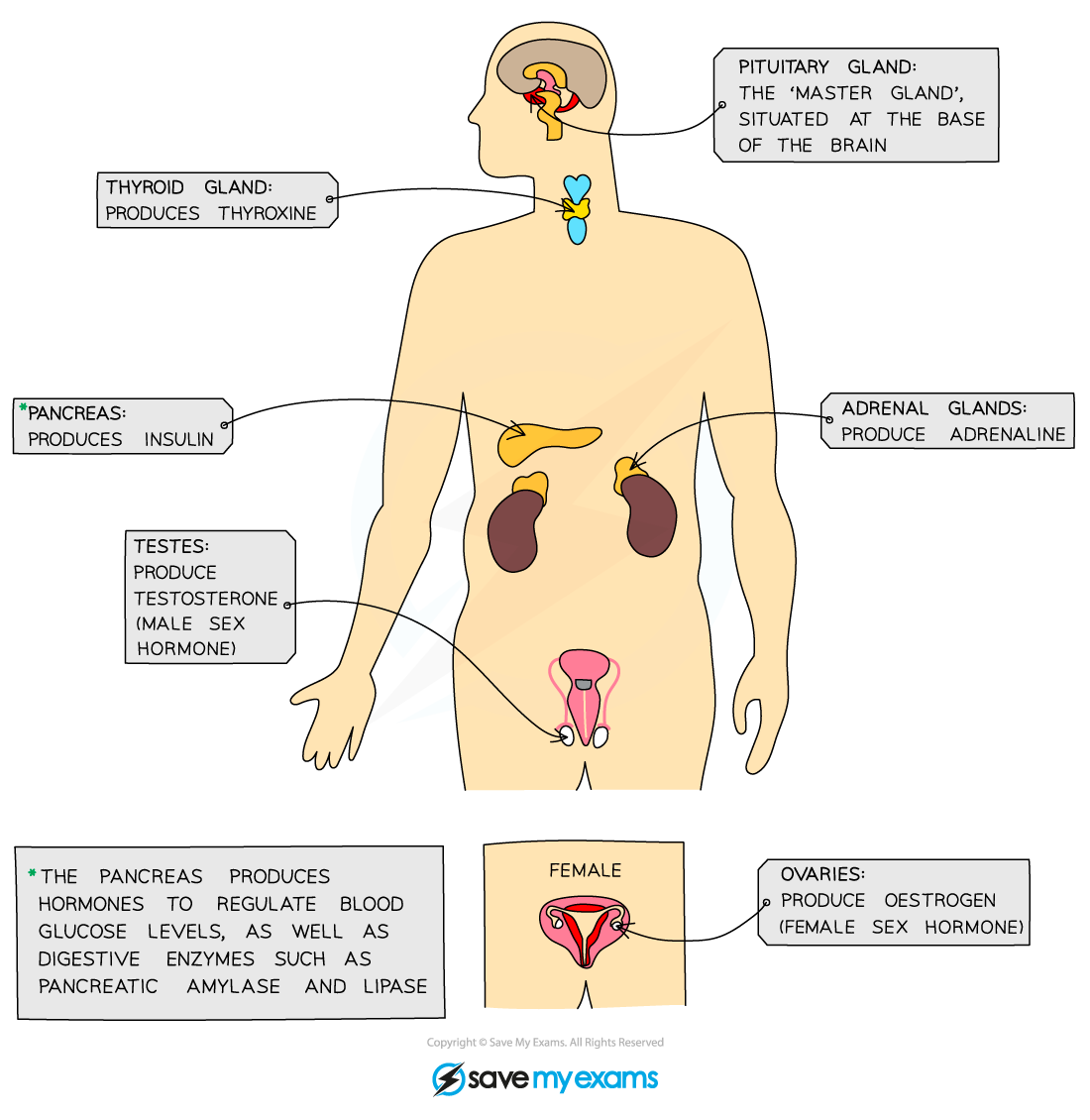
The major endocrine glands in the body
- Endocrine glands have a good blood supply as when they make hormones they need to get them into the bloodstream (specifically the blood plasma) as soon as possible so they can travel around the body to the target organs to bring about a response
- Hormones only affect cells with receptors that the hormone can bind to
- These are either found on the cell surface membrane, or inside cells
- Receptors have to be complementary to hormones for there to be an effect
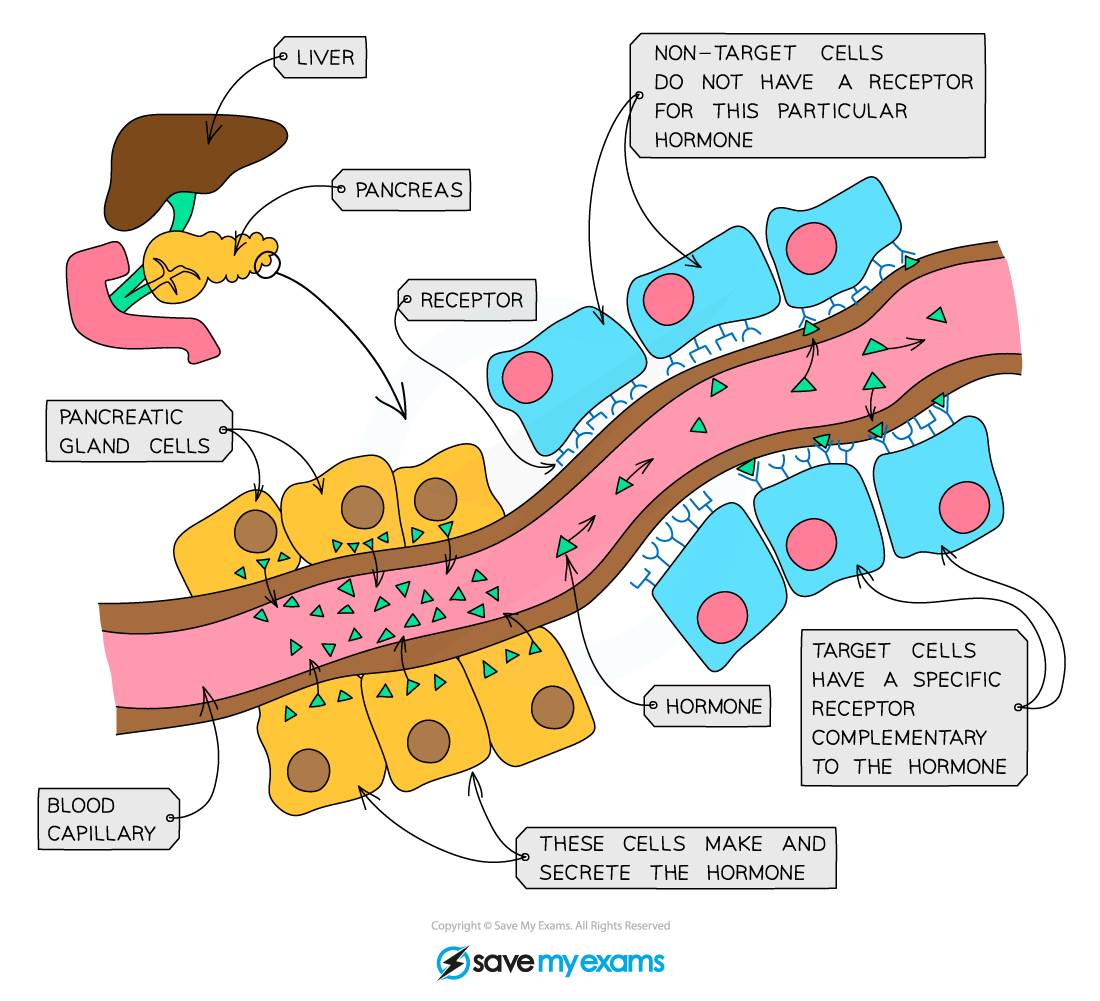
Hormones are synthesized and released into the bloodstream from a gland (such as the pituitary gland) and circulate in the bloodstream, affecting target cells
The Importance of Homeostasis: Temperature & pH
- Homeostatic mechanisms help organisms to keep their internal body conditions within restricted limits
- Two key factors that need to be controlled include:
- Temperature
- pH
- A stable core temperature and blood pH are vital for enzyme activity
- If the temperature or pH of the tissue fluid surrounding cells is too high or too low it can negatively affect the rate of important enzyme-controlled reactions
Temperature
pH
- All enzymes have an optimum pH or a pH at which they operate best
- Enzymes are denatured at extremes of pH
- Hydrogen and ionic bonds hold the tertiary structure of the protein (ie. the enzyme) together
- Below and above the optimum pH of an enzyme, solutions with an excess of H+ ions (acidic solutions) and OH– ions (alkaline solutions) can cause these bonds to break
- This alters the shape of the active site, which means enzyme-substrate complexes form less easily
- Eventually, enzyme-substrate complexes can no longer form at all
- At this point, complete denaturation of the enzyme has occurred
- Where an enzyme functions can be an indicator of its optimal environment:
- Eg. pepsin is found in the stomach, an acidic environment at pH 2 (due to the presence of hydrochloric acid in the stomach’s gastric juice)
- Pepsin’s optimum pH, not surprisingly, is pH 2
The Importance of Homeostasis: Blood Glucose Concentration
- Another key factor that must be controlled within mammals is the concentration of glucose in the blood
- The amount of glucose present in the blood affects the water potential of the blood and the availability of respiratory substrate for cells
- The normal glucose concentration for human blood is roughly 90mg per 100cm3
- A sufficient amount of circulating glucose is essential for cellular respiration
- Brain cells can become rapidly damaged or die if they do not receive a sufficient supply of glucose
- Alternatively, if the blood glucose concentration is too high then it will have a dramatic effect on the water potential of the blood
转载自savemyexams

最新发布
© 2026. All Rights Reserved. 沪ICP备2023009024号-1









