- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
AQA A Level Biology复习笔记5.4.1 Nutrient Cycles
Nutrient Cycles
- Living organisms require nutrients from their environments for growth and other processes (e.g. reproduction)
- These nutrients are then returned to the environment when organisms produce waste or die and decompose
- This is due to the waste products and dead organisms being digested (decomposed) by microorganisms
- The products of this decomposition are available to plants as nutrients in the soil
- These plants can then sustain organisms in higher trophic levels (consumers)
- In stable communities, the processes that remove nutrients (e.g. plant growth) are balanced by the processes that return these nutrients (e.g. decomposition of dead plants and animals)
- This means these nutrients are constantly being cycled in ecosystems
- Two examples of these nutrient cycles are:
- The nitrogen cycle
- The phosphorous cycle
The nitrogen cycle
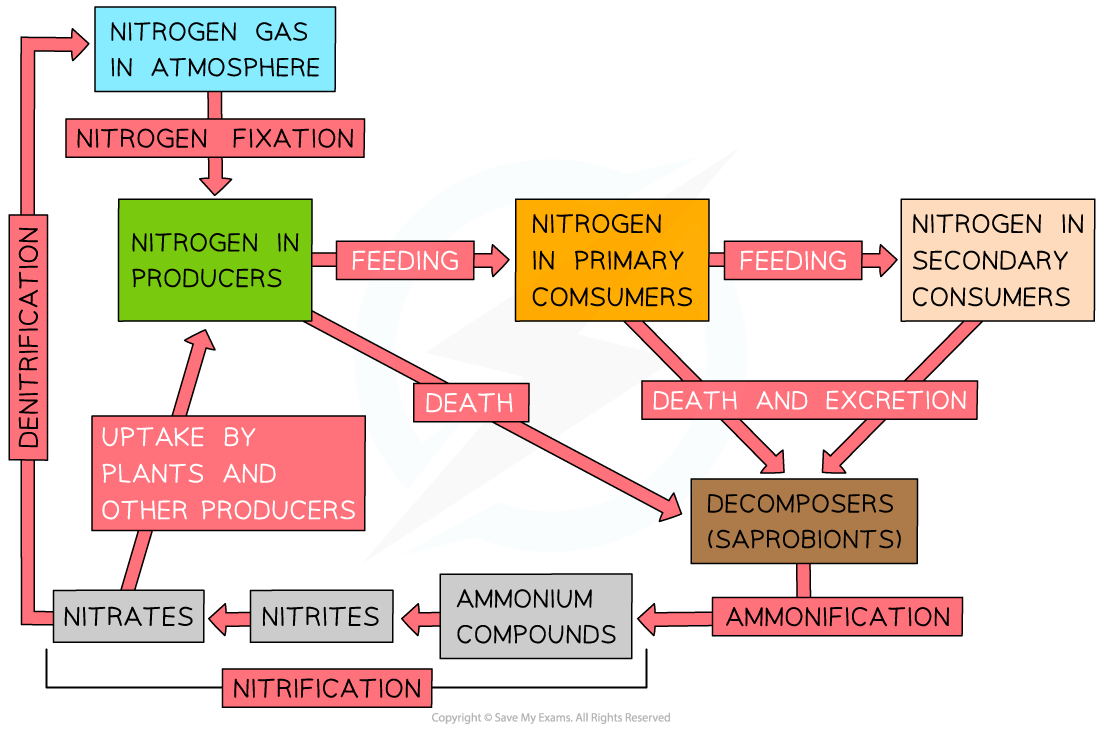
- The nitrogen cycle shows how nitrogen is recycled in ecosystems
- Plants and animals require nitrogen in order to produce proteins and nucleic acids (DNA and RNA)
- About 78% of the atmosphere is actually nitrogen gas but plants and animals cannot access the nitrogen in this gaseous form
- Instead, they rely on certain bacteria to convert the nitrogen gas into nitrogen-containing compounds, which can be taken up by plants
- The nitrogen cycle shows this conversion, as well as how the nitrogen in the nitrogen-containing compounds is then passed between trophic levels or between living organisms and the non-living environment
The role of bacteria in the nitrogen cycle
- There are four key processes in the nitrogen cycle that are carried out by different types of bacteria
- Nitrogen fixation:
- Atmospheric nitrogen gas is converted into nitrogen-containing compounds
- This biological nitrogen fixation is carried out by nitrogen-fixing bacteria such as Rhizobium
- The bacteria convert nitrogen into ammonia, which forms ammonium ions (in solution) that can then be used by plants
- These nitrogen-fixing bacteria are found inside the root nodules (small growths on the roots) of leguminous plants such as peas, beans and clover
- The bacteria have a symbiotic (mutually beneficial) relationship with these plants - the bacteria provide the plants with nitrogen-containing compounds and the plants provide the bacteria with organic compounds such as carbohydrates
- Ammonification:
- Nitrogen compounds in waste products (e.g. urine and faeces) and dead organisms are converted into ammonia by saprobionts (a type of decomposer including some fungi and bacteria)
- This ammonia forms ammonium ions in the soil
- Nitrification:
- The ammonium ions in the soil are converted by nitrifying bacteria into nitrogen compounds that can be used by plants, known as nitrates
- Initially, nitrifying bacteria such as Nitrosomonas convert ammonium ions into nitrites
- Different nitrifying bacteria such as Nitrobacter then convert these nitrites into nitrates
- Denitrification:
- Denitrifying bacteria use nitrates in the soil during respiration
- This process produces nitrogen gas, which returns to the atmosphere
- This process occurs in anaerobic conditions (when there is little or no oxygen available, such as in waterlogged soil)
The nitrogen cycle
The phosphorus cycle
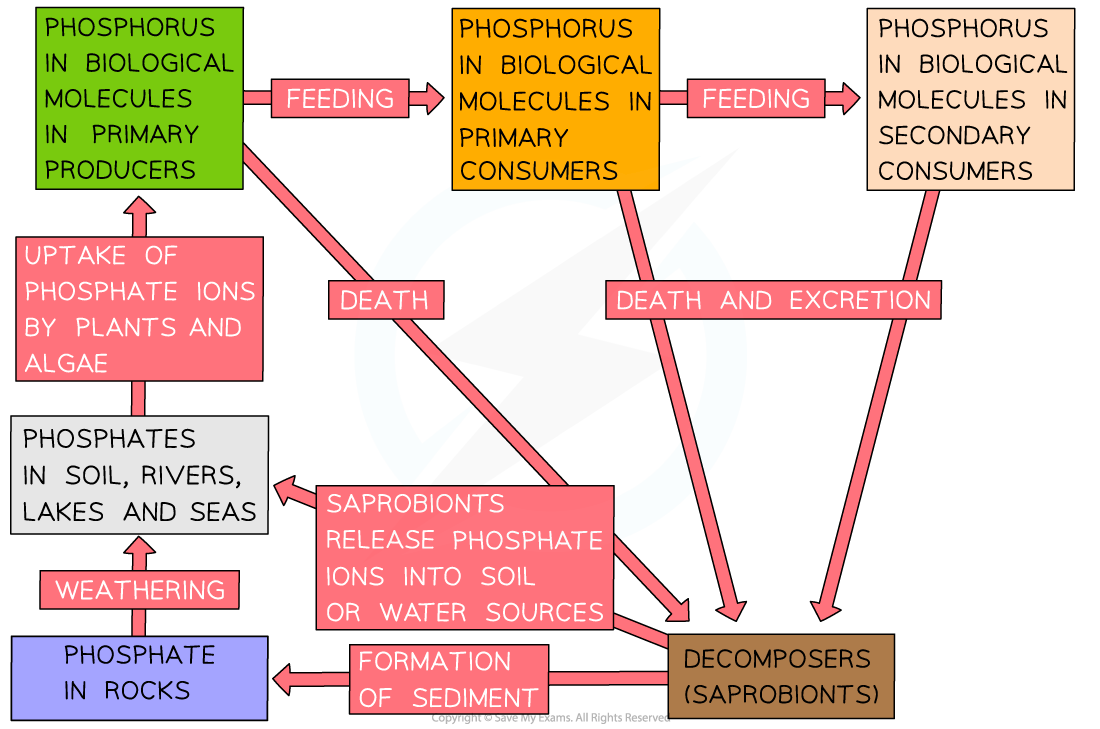
- The phosphorus cycle shows how phosphorus is recycled in ecosystems
- Plants and animals require phosphorus in order to produce certain biological molecules such as phospholipids (for cell membranes), nucleic acids (DNA and RNA) and ATP
- The phosphorus cycle includes the following processes:
- Phosphorus in rocks is slowly released into the soil and into water sources in the form of phosphate ions (PO₄³⁻) by the process of weathering (the slow breaking down and erosion of rocks over time)
- Phosphate ions are taken up from the soil by plants through their roots or absorbed from water by algae
- Phosphate ions are transferred to consumers during feeding
- Phosphate ions in waste products and dead organisms are released into the soil or water during decomposition by saprobionts
- The phosphate ions can now be taken up and used once again by producers or may be trapped in sediments that, over very long geological time periods may turn into phosphorus-containing rock once again
The phosphorus cycle
转载自savemyexams

早鸟钜惠!翰林2025暑期班课上线

最新发布
© 2025. All Rights Reserved. 沪ICP备2023009024号-1








