- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
AQA A LEVEL BIOLOGY复习笔记1.3.1 Amino Acids & the Peptide Bond
Amino Acids & the Peptide Bond
Proteins
Proteins are polymers (and macromolecules) made of monomers called amino acids
The sequence, type and number of the amino acids within a protein determines its shape and therefore its function
Proteins are extremely important in cells because they form all of the following:
Enzymes
Cell membrane proteins (eg. carrier)
Hormones
Immunoproteins (eg. immunoglobulins)
Transport proteins (eg. haemoglobin)
Structural proteins (eg. keratin, collagen)
Contractile proteins (eg. myosin)
Amino acid
Amino acids are the monomers of proteins
There are 20 amino acids found in proteins common to all living organisms
The general structure of all amino acids is a central carbon atom bonded to:
An amine group -NH2
A carboxylic acid group -COOH
A hydrogen atom
An R group (which is how each amino acid differs and why amino acid properties differ e.g. whether they are acidic or basic or whether they are polar or non-polar)
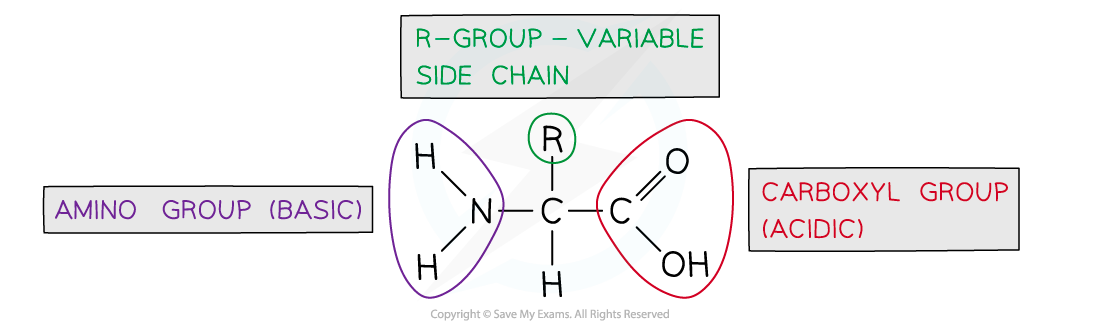
The generalised structure of an amino acid
Peptide bond
In order to form a peptide bond a hydroxyl (-OH) is lost from the carboxylic group of one amino acid and a hydrogen atom is lost from the amine group of another amino acid
The remaining carbon atom (with the double-bonded oxygen) from the first amino acid bonds to the nitrogen atom of the second amino acid
This is a condensation reaction so water is released
Dipeptides are formed by the condensation of two amino acids
Polypeptides are formed by the condensation of many (3 or more) amino acids
A protein may have only one polypeptide chain or it may have multiple chains interacting with each other
During hydrolysis reactions, the addition of water breaks the peptide bonds resulting in polypeptides being broken down to amino acids
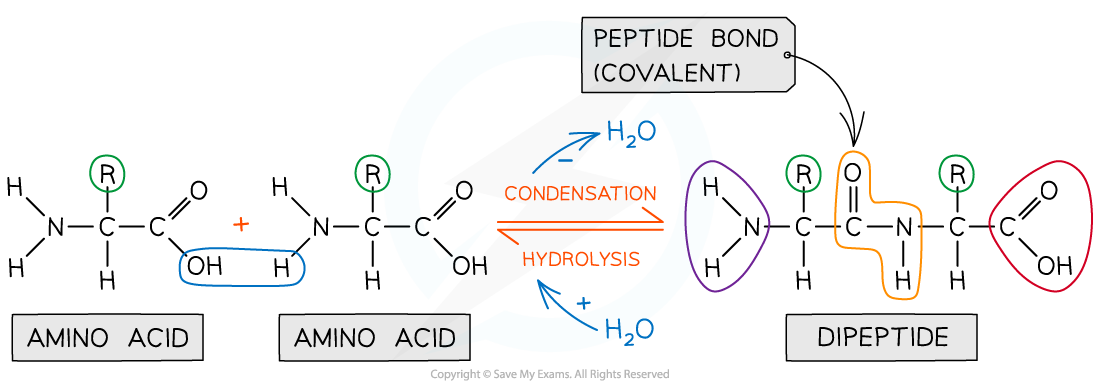
Amino acids are bonded together by covalent peptide bonds to form a dipeptide in a condensation reaction
转载自savemyexams


最新发布
© 2025. All Rights Reserved. 沪ICP备2023009024号-1








