- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
IBDP化学课程真题讲解(3)
通常而言,IBDP 化学被视作一门比较难的学科。但对于一些小伙伴而言,IB化学却并不那么难。这两者并不矛盾。今天带着大家一起看一道IBDP化学课程的真题:选择题(HIGHER LEVEL PAPER 1)。这道题取自于IBDP chemistry specimen paper。
题目1:What is the overall charge on the complex ion formed by iron(II) and six cyanide ions, CN-?
A. 4+
B. 4–
C. 8–
D. 8+
题目难度:简单。
考点分析:考查complex ion
题目分析:首先根据题意1个iron(II)结合6个cyanide ions,列式计算:(+2)+(-1)*6=-4,所以答案选B。
正确答案:B
题目2:Which property increases down group 1 of the periodic table?
A. Melting point
B. First ionization energy
C. Atomic radius
D. Electronegativity
题目难度:简单。
考点分析:考查periodic table的trend
题目分析:down group 1 of the periodic table, atomic radius increases, melting point decreases, first ionization energy decreases, electronegativity decreases(电负性降低,金属性升高)。
正确答案:C
题目3: One of the main constituents of acid deposition is sulfuric acid. This acid is formed from the sulfur dioxide pollutant.A mechanism proposed for its formation is:
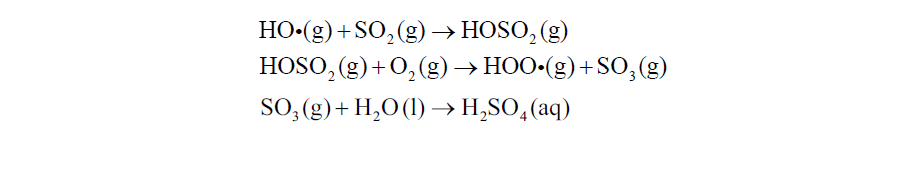
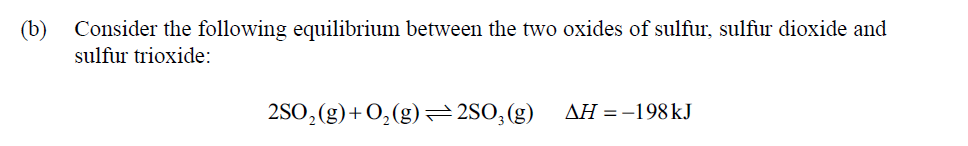
(c) Sketch the potential energy profile for the forward reaction in part (b) to show the effect of a catalyst on the activation energy.
题目难度:简单。
考点分析:考查energy profile
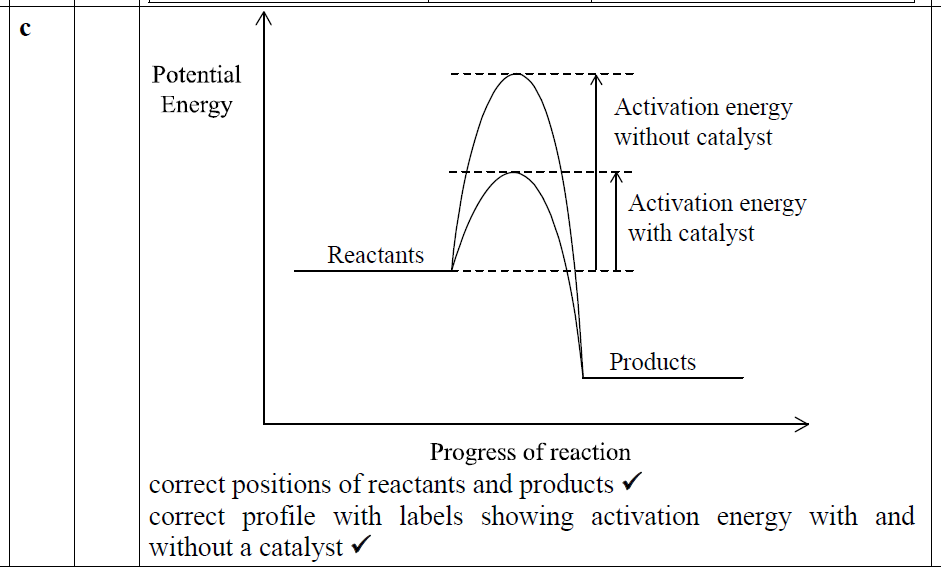
题目分析:part b的反应forward是放热的,所以products的能量低于reactants,然后再把activation energy给画上即可(有催化剂的矮,无催化剂的高)。
正确答案:
扫码添加翰林顾问老师,可一对一制定国际课程规划
【免费领取】IBDP/IB 备考资料合集~


最新发布
© 2025. All Rights Reserved. 沪ICP备2023009024号-1









