- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
IBDP化学课程真题讲解(2)
通常而言,IBDP 化学被视作一门比较难的学科。但对于一些小伙伴而言,IB化学却并不那么难。这两者并不矛盾。今天带着大家一起看一道IBDP化学课程的真题讲解(HIGHER LEVEL PAPER 2)。这道题取自于IBDP chemistry specimen paper。不多说,直接上题。
题目1:Two IB students carried out a project on the chemistry of bleach.
(b) Some of the group 17 elements, the halogens, show variable valency.
(i) Deduce the oxidation states of chlorine and iodine in the following species. [1]
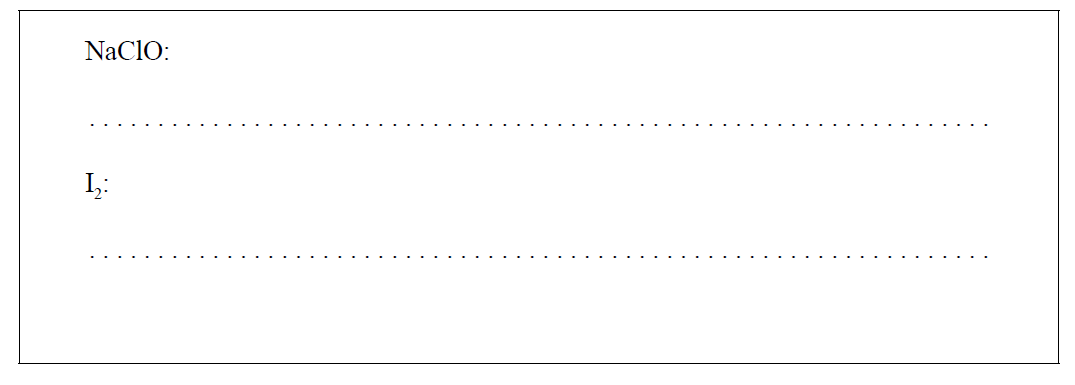
(ii) Deduce, with a reason, the oxidizing agent in the reaction of hypochlorite ions with iodide ions in part (a). [1]
(iii) From a health and safety perspective, suggest why it is not a good idea to use hydrochloric acid when acidifying the bleach. [1]
题目难度:简单。
考点分析:考查group 17 elements的相关性质
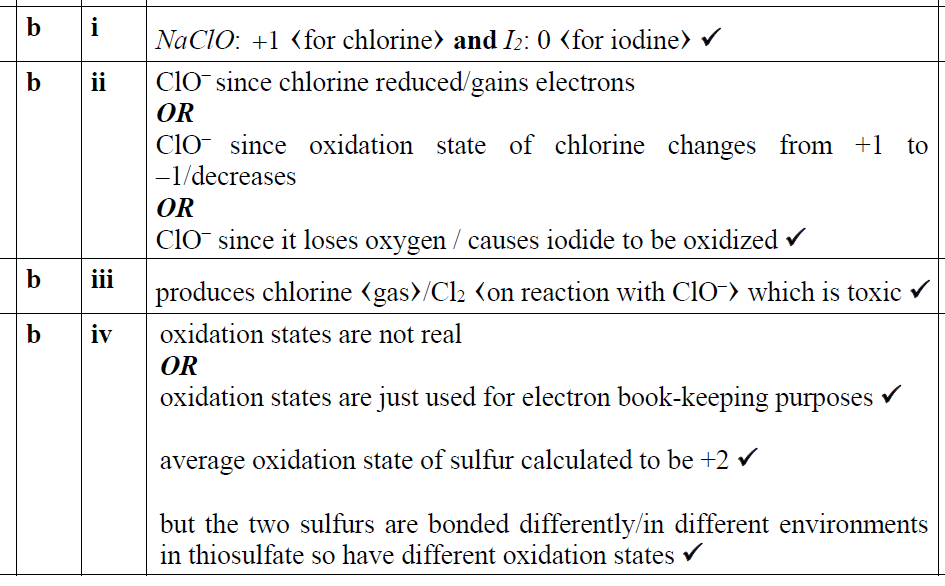
题目分析:第1问Cl是+1,I是-1;第2问oxidizing agent gains electrons,所以hypochlorite ions是oxidizing agent;第3问因为会产生有毒的氯气;第4问参看下面答案就很容易理解了。
正确答案:
题目2:Two IB students carried out a project on the chemistry of bleach.
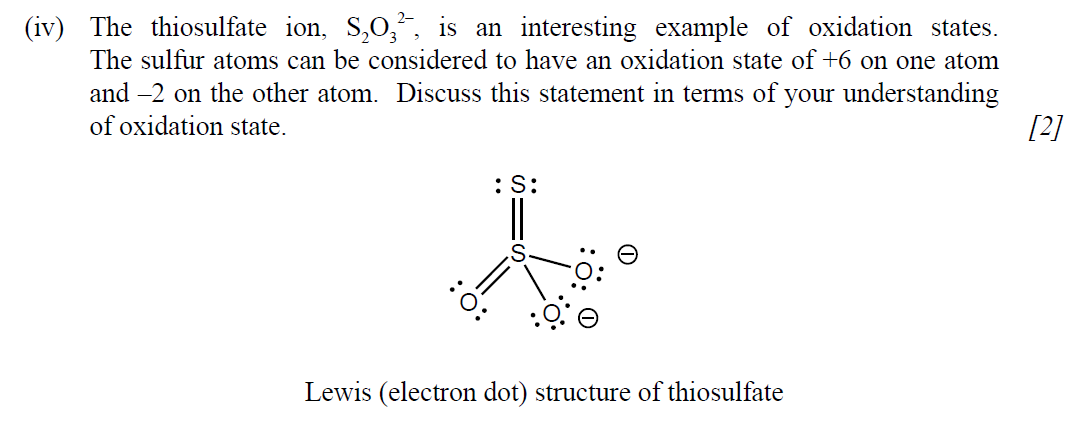
(i) State the condensed electron configuration of sulfur. [1]
(ii) Deduce the orbital diagram of sulfur, showing all the orbitals present in the diagram. [1]
题目难度:简单。
考点分析:考查electron configuration和orbital diagram
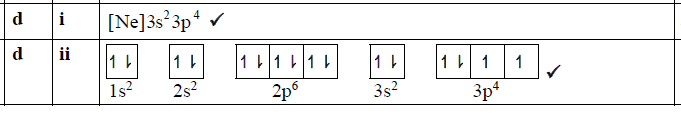
题目分析:S最外层有6个电子,其中3s有2个,3p有4个;3p轨道有3个,其中1个轨道有2个电子,另外2个轨道各1个电子。
正确答案:
扫码添加翰林顾问老师,可一对一制定国际课程规划
【免费领取】IBDP/IB 备考资料合集~


最新发布
© 2026. All Rights Reserved. 沪ICP备2023009024号-1









