- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
2022年UKChO化学竞赛即将开考,2021年UKChO竞赛获奖论文公布
2022年UKChO化学学术活动即将开考,正在备战的同学一定不要忘记从近几年的论文中找到灵感,刷题有做题的感受,查缺补漏是非常重要的,2021年UKChO学术活动获奖论文,一起来看看,还有更多论文等你来领!
扫码免费领取近几年【UKChO学术活动获奖论文及试题/答案】!
还有名师在线辅导,答疑解惑,每周都有的讲座,助力高分锦囊,尽在其中!

53rd INTERNATIONAL
CHEMISTRY OLYMPIAD
2021
UK Round One
STUDENT QUESTION BOOKLET
* * * * *
■ The time allowed is two hours.
■ Attempt all six questions.
■ Write your answers in the student answer booklet.
■ Write only the essential steps of your calculations in the answer booklet.
■ Always give the appropriate unit and number of significant figures.
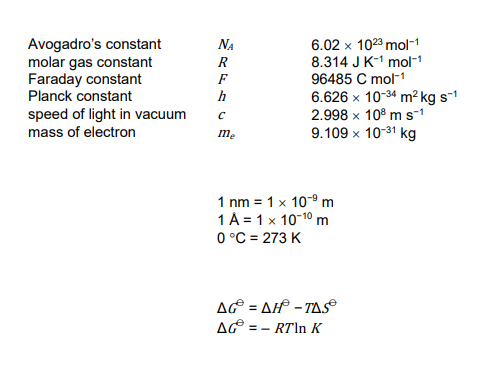
■ The final pages of this question booklet include a copy of the periodic table and some useful physical constants and quantities.
■ Do NOT write anything in the right-hand margin of the answer booklet.
■ The marks available for each question are shown below. These may be helpful when dividing your time between questions.
| Question | 1 | 2 | 3 | 4 | 5 | 6 | Total |
| Marks Available |
9 | 9 | 16 | 13 | 21 | 17 | 85 |
Some of the questions will contain material you will not be familiar with. However, you should be able to work through the problems by applying the skills you have learnt as a chemist. There are different ways to approach the tasks – even if you cannot complete certain parts of a question, you may find later parts straightforward.
This resource was downloaded from https://rsc.li/2WmGF2V。
1. This question is about life on Venus
In 2020, a controversial research paper announced the detection of phosphine (PH3) in the atmosphere of Venus. On Earth, phosphine typically comes from either biological processes or industrial processes and other than this is not produced ‘naturally’. Its possible presence on Venus indicates a potential biological origin, although the conditions on Venus are very different to Earth, with temperatures up to 698 K, pressures up to 95 atm and clouds of sulfuric acid.
(a) Draw the shape of phosphine.
Phosphine does not accumulate in Earth’s atmosphere as it reacts with oxygen to produce phosphoric acid (H3PO4). The atmosphere of Venus does not contain oxygen.
(b)
(i) State the oxidation states of phosphorus in phosphine and phosphoric acid.
(ii) Write an equation for the reaction of phosphine with oxygen to make phosphoric acid.
There are several methods to produce phosphine that do not require living organisms. These processes do not occur naturally on the Earth’s surface because the starting materials are not found or the conditions are not suitable.
Write equations for the following reactions which all produce phosphine.
(c)
(i) The reduction of phosphorus trichloride with lithium hydride.
(ii) The hydrolysis of calcium phosphide.
(iii) The high temperature disproportionation of phosphorous acid (H3PO3).
Another simple molecule often detected in space is methanal. Methanal is highly reactive, so any produced on Venus would be quickly consumed. Phosphine reacts with methanal under acidic conditions.
In the presence of sulfuric acid, phosphine and methanal react to give a salt of molar mass
406.272 g mol −1 . The cation of this salt has an overall charge of +1. The 13C NMR of this salt has only one signal. The 1H NMR of this salt shows two signals, one of which exchanges with D2O.
(d) (i) Write the formula of the anion.
(ii) Draw the structure of the cation.
2. This question is about capturing carbon

Carbon capture is a key technology for reducing greenhouse gas emissions.
One of the most promising carbon capture technologies, calcium looping, involves cycling between calcium carbonate and calcium oxide to capture CO2. The calcium carbonate used in this process most commonly comes from limestone.
The captured carbon dioxide can then either be stored underground or used, for example in producing synthetic fuels.
In calcium looping, CO2 is captured from flue (exhaust) gases. A diagram of this cycle is shown below.
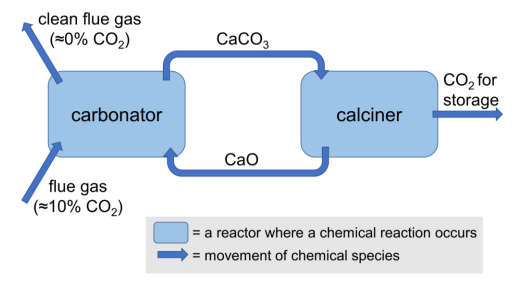
(a) Write an equation for the main reaction in the carbonator.
(b) Write an equation for the main reaction in the calciner.
One of the impurities in flue gases is sulfur dioxide from the combustion of sulfur-containing fuels. This can react in the carbonator to form an unreactive impurity, which can accumulate over successive cycles and reduce the efficiency of the process.
(c) Write an equation for the formation of sulfur dioxide from the combustion of hydrogen sulfide (H2S).
(d) Write an equation for the reaction of sulfur dioxide with calcium carbonate and oxygen to give two products.
Direct air capture is a process which takes carbon dioxide directly from the air therefore avoiding any sulfur dioxide contamination. However, this process can be expensive to run due to the energy required to overcome thermodynamic barriers.
A scheme for a direct air capture process based on calcium looping is shown below.
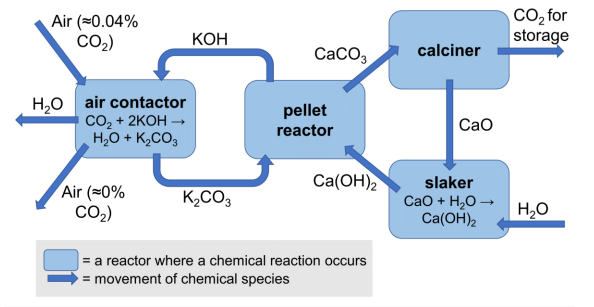
(e) Write an equation for the reaction in the pellet reactor.
(f) Calculate the standard enthalpy change for the reaction which occurs in the air contactor.
Standard enthalpy change of formations, ΔfH⦵, at 298 K in kJ mol−1:
CaO −635.1
CaCO3 −1206.9
Ca(OH)2 −986.1
KOH −424.8
K2CO3 −1151.2
CO2 −393.5
H2O −285.8
(g) Tick the correct statement that describes the standard enthalpy change at 298 K for the sum of the reactions in the four reactors.
(h) Tick the two correct statements about the entropy changes in this process.
3. This question is about levulinic acid
In 2008 Arsenal F.C. midfielder Mathieu Flamini founded GF Biochemicals, which was the first company to mass produce levulinic acid. Levulinic acid is a versatile chemical that can be used to make pharmaceuticals, plastics and fuels. Levulinic acid can be made from renewable resources such as the sugar fructose.
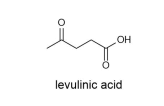
(a) Tick the names of the functional groups present in levulinic acid in the answer booklet.
(b) Give the molecular formula for levulinic acid.
Fructose exists in equilibrium between its linear form and its cyclic form. This equilibrium mixture can be converted to levulinic acid as follows.
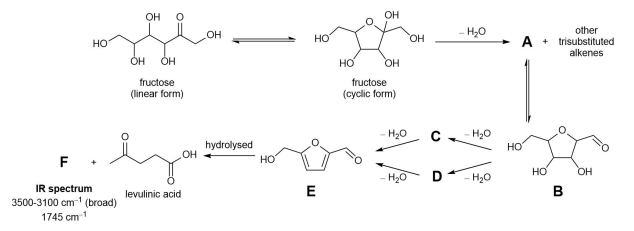
(c) Circle in the answer booklet the two atoms in the linear form of fructose which become bonded in the cyclic form.
Elimination of a molecule of water from the cyclic form of fructose forms compound A and several other trisubstituted alkenes. The alkene is formed due to loss of a hydrogen and a hydroxyl group (OH group) from adjacent carbon atoms as shown on the right.
If the alkene that is formed has an OH substituent, then it can isomerise as shown on the right.
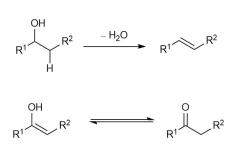
(d)
(i) Draw the structure of alkene A, which can isomerise to form B.
(ii) Draw the structure of the other trisubstituted alkenes that could also be formed.
Compound B can be converted into compound E via the elimination of two more molecules of water. This can occur via intermediate compounds C or D.
(e) Draw the structure of compounds C and D.
Compound E can by hydrolysed to levulinic acid and by-product compound F.
(f) Draw the structure of compound F.
The hydrogenation of levulinic acid gives a green and sustainable route to several different chemicals used in perfumes and flavouring. One catalyst for this hydrogenation is the octahedral complex of formula [RuH2(CO)(PPh3)3]. This complex is named dihydridocarbonyltris(triphenylphosphine)ruthenium(Z), where Z is the oxidation state of ruthenium.
(g) State the value of Z.
The stereochemistry of the catalyst [RuH2(CO)(PPh3)3] is shown.
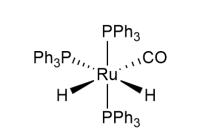
(h) Draw the other stereoisomers of this complex. Tick to indicate whether the stereoisomer shown and each stereoisomer you have drawn has an enantiomer. For a pair of enantiomers, only draw one of the pair.
This catalyst can be analysed by 31P NMR. 31P is 100% abundant and is a spin ½ nucleus like 1H. The 31P NMR is run in a way that means no coupling between 31P and 1H is observed. The only coupling which is observed is between 31P nuclei in different environments.
(i) What is observed in the 31P NMR spectrum of this catalyst? Tick the correct answer.
In the 1H NMR spectrum, the two hydrides appear at negative chemical shifts and have complex coupling patterns. The couplings observed for these signals arise from coupling through two bonds to either 1H or 31P, (i.e. either 1H–Ru– 1H, or 1H–Ru–31P) and are measured in Hz. The coupling constant between two nuclei that are trans is usually larger than between two nuclei that are cis.
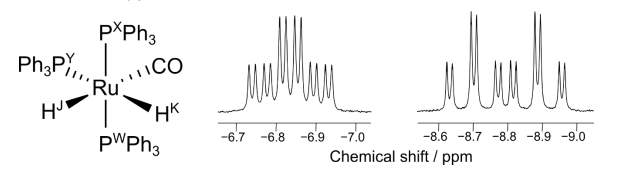
The signal at −6.83 ppm is a ‘triplet of doublets of doublets’. The signal is split into a triplet with the largest coupling constant of 31 Hz, further split into a doublet with coupling constant of 15 Hz, further split into a doublet with coupling constant of 6 Hz.
The signal at −8.80 ppm is a ‘doublet of triplets of doublets’. The signal is split into a doublet with the largest coupling constant of 74 Hz, further split into a triplet with coupling constant of 28 Hz, further split into a doublet with coupling constant of 6 Hz.
(j) Complete the table to assign the values of the coupling constants in Hz to the following pairs of nuclei (HJ–HK, HJ–PW, HJ–PX, HJ–PY, HK–PW, HK–PX, HK–PY).
Q4 This question is about ‘social distancing’ within molecules
The COVID-19 pandemic has introduced us to social distancing. This question is about how the arrangement of molecules in 3D space often results in parts of those molecules being as far from each other as possible.
In the molecule ethane, rotation around the central C–C bond means that the relative position of the hydrogen atoms in 3D space can change. This rotation happens rapidly at room temperature. The different 3D arrangements are called conformers (or conformational isomers).
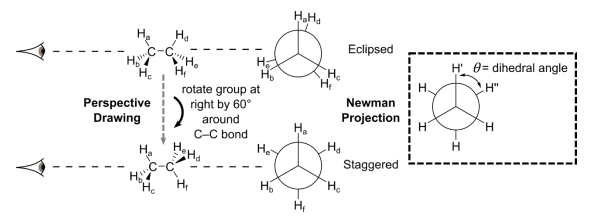
These conformers are represented using a Newman projection. A Newman projection is a view of the molecule along the C–C bond represented by a circle. The bonds to hydrogen made by the carbon atom at the front are shown in front of the circle. The bonds made to hydrogen by the carbon atom at the back are shown behind the circle. This view clearly shows the angle between the C−H bonds on the front carbon and the C−H bonds on the back carbon. This angle is called the dihedral angle, and the dihedral angle θ between H’ and H’’ is shown above. In ethane, the eclipsed conformation where θ = 0° is higher in energy than the staggered conformation where θ = 60°, as the hydrogens are less ‘socially distanced’.
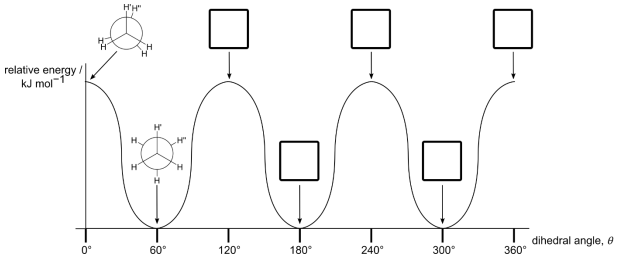
(a) The relative energy of the different conformations for a complete 360° rotation around the C–C bond in ethane is shown on the diagram above. For the five dihedral angles shown on the graph, indicate whether ethane is in an eclipsed conformation (write E in the box), or a staggered conformation (write S in the box).
Although θ can take any value between 0° and 360° for rotation around a bond, we often consider just six conformations (three staggered and three eclipsed). The diagram shows the relative energy of the conformations for rotation around the C2−C3 bond in methylbutane.
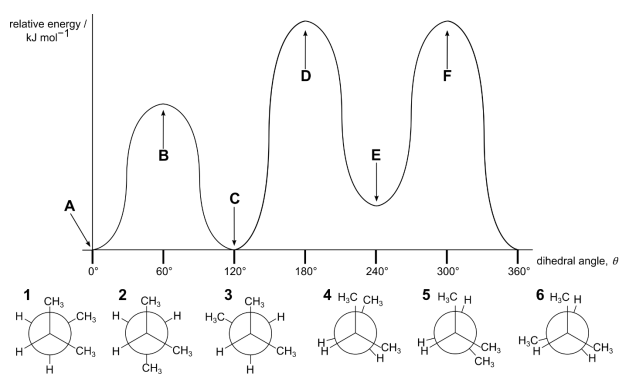
(b) Match the six Newman projections of methylbutane (1−6) with the points A−F on the energy diagram. Write only one number in each box.
The energy difference between conformations can be used to approximate the percentage of time a molecule will be found in each conformation by treating the interconversion between conformations as equilibria.
For butane, we will assume that the molecule is always in one of the three staggered
conformations: gauche 1 (G1), antiperiplanar (AP), or gauche 2 (G2).
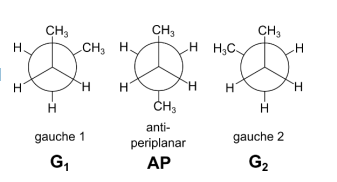
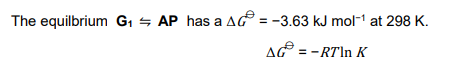
(c)
(i) Complete the table in the answer booklet by calculating the Gibbs energy change, ΔG⦵, and the equilibrium constant, K, for each of the three equilibria at 298 K.
G1 ⇋ AP AP ⇋ G2 G2 ⇋ G1
(ii) Hence, calculate the percentage of time that butane is in the anti-periplanar conformer AP.
Acetylcholine is a neurotransmitter released by motor neurons to activate muscles.
Six Newman projections of different conformations of acetylcholine are shown. The ethanoate ester group is abbreviated as OAc and the methyl groups are abbreviated as Me.
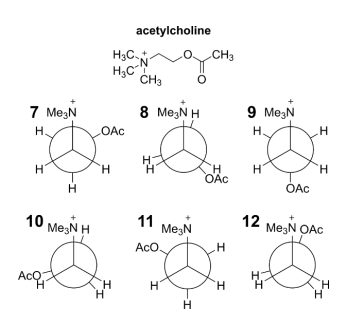
(d) Group these conformations into sets of equal energy.
For example you could write:
(7 = 8 = 9)
(10 = 11)
12
Include all conformations in your answer.
Although acetylcholine can adopt many conformations in solution, when it binds in muscle receptors it has to adopt a particular conformation. Drugs to treat various diseases seek to block acetylcholine binding.
Many of these drugs are more rigid molecules, where the angle between the (NMe3)+ and the OAc groups cannot freely change. The drugs which most closely resemble the 3D shape of bound acetylcholine usually bind most strongly in the receptors and so are the most effective blockers.
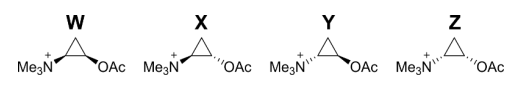
The cyclopropane rings in molecules W, X, Y and Z are planar and have very little conformational flexiblity. W, X, Y and Z were investigated as acetylcholine blockers.
(e) For each conformation of acetylcholine 7 – 12, tick whether they are mimicked by any of the cyclopropanes W, X, Y or Z. Some conformations may have more than one mimic and some conformations may have no mimics.
5. This question is about Donald Trump and the coronavirus
Donald Trump suggested several strategies for preventing or treating a coronavirus infection, which medical experts have disagreed with. These include shining UV light inside the body and injecting disinfectant. In May 2020, Trump said that he was taking hydroxychloroquine. In October 2020, it was reported that he had tested positive for COVID-19.
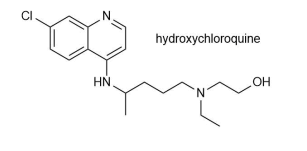
Hydroxychloroquine may be synthesised by the following route. Note in this question not all by-products are shown in the reaction schemes.
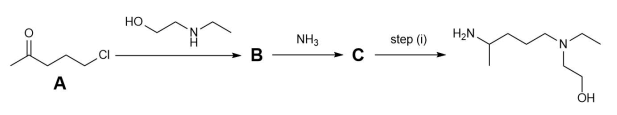
(a) Give the IUPAC name for starting material A.
(b) Draw the structures of B and C.
(c) Tick in the answer booklet the reagents/conditions required to perform step (i).
Another starting material in the synthetic route is benzene.
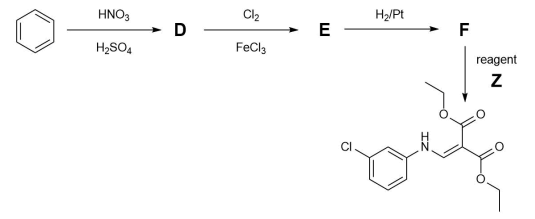
(d) Draw the structures of D, E and F.
Reagent Z is synthesised as shown below. When treated with a base diethyl malonate forms anion V−. When treated with an acid triethyl orthoformate forms cation W+. Cation W+fragments, losing ethanol to give cation X+. Anion V− and cation X+ then combine to form intermediate Y, which loses a further molecule of ethanol to form Z.
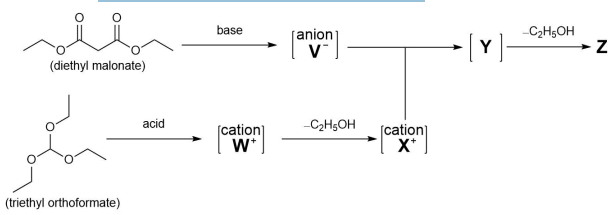
(e) Draw the structures of anion V−, cation W+, cation X+, intermediate Y, and reagent Z.The final part of the synthesis is shown below.
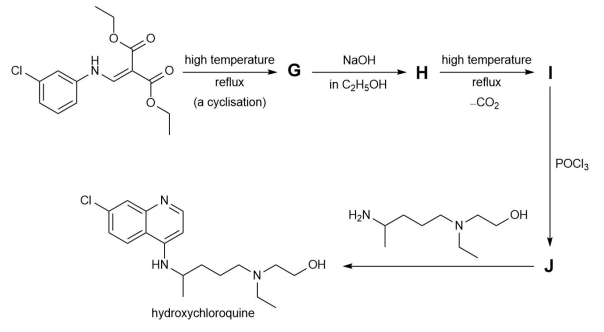
(f) Draw the structures of G, H, I, and J.
6. This question is about fluorides of xenon
At the time of its discovery by Scottish chemist Sir William Ramsay, the noble gas xenon was considered to be inert. It has since been discovered that xenon will react with strong oxidants. For example, xenon reacts with fluorine gas, forming a series of fluorides, XeF2, XeF4 and XeF6.
(a) Write an equation for the formation of xenon tetrafluoride.
The structure of xenon tetrafluoride has six electron pairs on xenon and therefore the structure is based on an octahedral configuration.
(b) Draw a dot-and-cross structure of xenon tetrafluoride.
(c) Draw the two possible three-dimensional arrangements of the electron pairs on xenon in xenon tetrafluoride and tick the one adopted.
(d) Draw the possible three-dimensional arrangements of the five electron pairs on xenon in xenon difluoride and tick the one adopted.
The kinetics of the formation of xenon difluoride from xenon and fluorine has been studied under various conditions. The following table shows the instantaneous reaction rates at 120 °C for different initial concentrations of the reagents. Assume the volume of the reaction vessel is constant.

(e) What is the rate equation for the formation of xenon difluoride?
The Arrhenius equation describes the relationship between the rate constant and temperature.
= e−/T
The uncatalysed reaction between xenon and fluorine to form XeF2 at a temperature T has a rate constant k, with collision frequency factor A and activation energy Ea. R is the universal gas constant.
When a nickel difluoride catalyst is added to the reaction mixture, the rate constant changes to kcat, with a different collision frequency Acat and activation energy Ecat. It is found that the catalysed reaction is 13 times faster at 120 °C and 23 times faster at 100 °C.
The change in activation energy ΔE = Ea − Ecat.
(f) Write an expression for the ratio kcat/k in terms of T, A, Acat, ΔE and any constants.
(g) Calculate the change in activation energy, ΔE, in kJ mol−1, assuming that the collision frequency factors do not depend on temperature.
The rate-determining step in the uncatalysed formation of xenon difluoride is the reaction of atomic fluorine with xenon, F(g) + Xe(g) → XeF(g). A more refined kinetic theory expresses the rate constant of this reaction as:
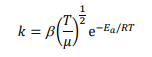
where β is a constant and µ is a reduced mass, neither of which depend on temperature. The rate constant has the following values at different temperatures:

(h) Determine the activation energy of this reaction using the data tabulated above.
When xenon was discovered in 1898 it was assigned a relative atomic mass of 128, which is mistakenly reported as 28 in the picture at the start of the question. The relative atomic mass has since been refined to its current value of 131.29. The rate constant depends on the reduced mass, , where:
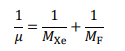
Xe and F are the atomic masses of xenon and fluorine in g mol−1 as given in the periodic table at the end of the paper.
(i) Determine the rate constant for the reaction F + Xe → XeF at 100 °C if the atomic mass of xenon was 28 g mol−1. Assume all other parameters remained unchanged.
Acknowledgements & References
Q1 The image is © Dr Ben Pilgrim
Phosphine gas in the cloud decks of Venus Nature Astronomy, 2020,
https://doi.org/10.1038/s41550-020-1174-4
Q2 The image is © Science Photo Library
A Process for Capturing CO2 from the Atmosphere Joule, 2018, 2, 1573-1594.
https://doi.org/10.1016/j.joule.2018.05.006
Q3 The image is © Getty Images
Clean Production of Levulinic Acid from Fructose and Glucose in Salt Water by Heterogeneous Catalytic Dehydration ACS Omega, 2020, 5, 14275-14282.
https://doi.org/10.1021/acsomega.9b04406
Solid acid catalyzed synthesis of furans from carbohydrates Catalysis Reviews, 2016, 58, 36-112.
https://doi.org/10.1080/01614940.2015.1099894
Combining Sustainable Synthesis of a Versatile Ruthenium Dihydride Complex with Structure Determination Using Group Theory and Spectroscopy Journal of Chemical Education, 2017, 94, 928-931.
https://doi.org/10.1021/acs.jchemed.6b00874
Q4 The image is © Shutterstock
Q5 The image is © Dr Ben Pilgrim
https://www.bbc.co.uk/news/world-us-canada-52399464
Q6 The image is © Dr George Trenins. The original public domain image was a caricature of Ramsay by Leslie Ward, in Vanity Fair in 1908. Ramsay was pointing to krypton in the original image that contained the wrong relative atomic mass. The image was edited for this paper so that Ramsay was pointing to xenon, but we did not correct the relative atomic mass.
Data is adapted from On the xenon-fluorine reactions Journal of Inorganic and Nuclear Chemistry, 1976, 28, 173-178.
https://doi.org/10.1016/0022-1902(76)80622-7
This work is published under the following Creative Commons License
Attribution-NonCommercial-ShareAlike CC BY-NC-SA
This license lets others remix, tweak, and build upon your work non-commercially, as long as they credit you and license their new creations under the identical terms.
Authors of 2020 UK Round One Paper (listed alphabetically)
Dr Helen Alonzi (St Bartholomew’s School)
Peter Bolgar (St Catharine’s College, University of Cambridge)
Chloe Francis (Royal Society of Chemistry)
Mark Jordan (Royal Society of Chemistry)
Sky Kang (Up Learn)
Dr JL Kiappes (Corpus Christi College, University of Oxford)
Dr Ben Pilgrim (University of Nottingham)
Dr Penny Robotham (The National Mathematics and Science College)
Richard Simon (Element Energy)
Dr Andy Taylor (King Edward VI Camp Hill School for Boys)
Dr Alex Thom (Girton College, University of Cambridge)
Dr George Trenins (ETH Zürich)
Physical Constants & Formulae
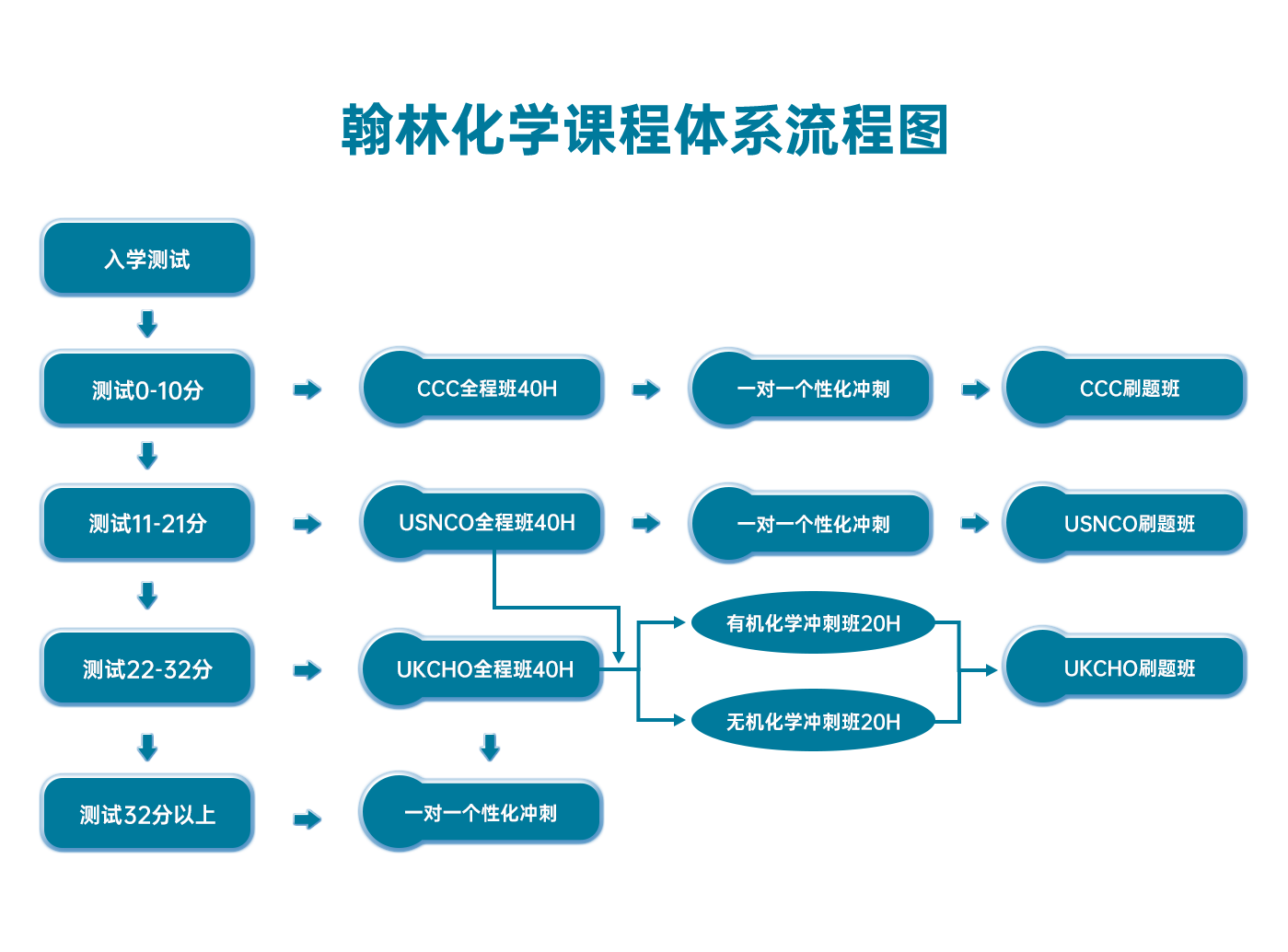
翰林化学学术活动课程体系流程图


最新发布
© 2025. All Rights Reserved. 沪ICP备2023009024号-1








