- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
IGCSE化学paper2重要考点
考试倒计时仅剩3天考前焦虑?
不必惊慌!
该会的你都会了!
6月12号paper 2会考哪些内容呢?我这里提供下考试的重点topic,没复习好的可要抓紧时间了。
来,上点干货,这些是考试重点内容:
atomic structurereactivity of metals
chemical test
organic
acid base and salt
calculation
energy change
bond energy
chromatography
elements, compounds and mixtures
the periodic table
rate of reaction
electrolysis
redox
equilibrium
gas in atmosphere
group 7
state of matter
group 1
titration
dot and cross digram
bonding and structure
crude oil
ammonia
formula equation
group 2
alkene
今天,我主要来说说chemical test部分。
按照大纲要求:
(1)describe tests for these gases:
• hydrogen 【‘pops’ with a lighted splint】
• oxygen 【relights a glowing splint】
• carbon dioxide 【gives a white ppt. with limewater】
• ammonia 【turns damp red litmus paper blue】
• chlorine 【bleaches damp litmus paper】
(2)describe how to carry out a flame test
【1. The technique is first of all to clean the end of a piece of platinum or nichrome wire by dipping it into clean hydrochloric acid and then placing it in a roaring Bunsen flame. This procedure should be repeated until the wire no longer produces a colour in the flame.2. The end of the wire should then be dipped into fresh hydrochloric acid and then into the solid sample under test.3. The end of the wire should then be placed into a non-roaring, non-luminous Bunsen flame.】
(3)know the colours formed in flame tests for these cations:• Li+ is red• Na+ is yellow• K+ is lilac
• Ca2+ is orange-red
• Cu2+ is blue-green
(4)test of cations
• NH4+ using sodium hydroxide solution and identifying the gas evolved 【放出的气体是NH3】
• Cu2+, Fe2+ and Fe3+ using sodium hydroxide solution 【Cu(OH)2, Fe(OH)2, Fe(OH)3颜色分别是blue, green, red-brown】
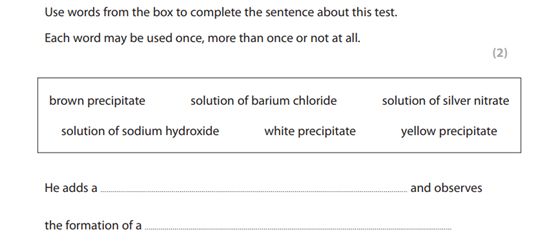
(5)test of anions• Cl–, Br– and I– using acidified silver nitrate solution 【AgCl, AgBr, AgI颜色分别是white, cream, yellow】
• SO42– using acidified barium chloride solution 【BaSO4是white precipitate】
• CO32– using hydrochloric acid and identifying the gas evolved. 【CO2 is released】
来,我们看道真题:
![]()


早鸟钜惠!翰林2025暑期班课上线

最新发布
© 2025. All Rights Reserved. 沪ICP备2023009024号-1








