- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
Edexcel化学往年真题考点详解(1)
马上就要到暑假了,同学们高兴之余也不要忘记学习哦,为了帮助同学们更好地巩固所学知识,今天我们通过几道真题,给大家回顾一下所学的重点知识。
1. Compounds A and B are isomeric alkenes.
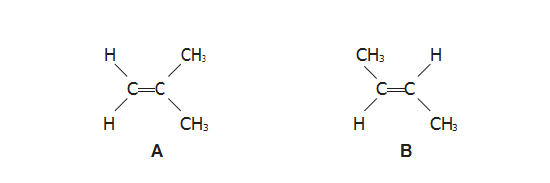
(a)(i) Name compound A.
【答案】:2-methylprop-1-ene
【分析】:这道题主要考察的是烃类的命名。
对于烃类(hydrocarbon)的命名我们的答题方法是:
第一步选主链,主链的选择原则是选含官能团(functional group)在内的最长的碳链为主链,本题中包含双键(double bond)在内的最长碳链有3个碳且含有双键所以他是一个propene。
第二步定编号,编号原则是官能团(functional group)和取代基(substituent group) 位次最小(官能团优先排序)。所以应从左边的碳开始排序,在二号碳的位置有一个甲基所以是2- methyl,双键起始于第一个碳所以是1-ene
所以,这道题的答案是 2-methylprop-1-ene
(ii) Give the molecular formula of compound B.
【答案】:C4H8
【分析】:回答这道题时,应体现出物质所有的元素以及所有元素所含原子的真实个数。
(iii) Explain why A and B are isomers.
【答案】:A and B have the same molecular formula but different structural formula
【分析】:这道题考察的是同分异构体的概念。
具有相同分子式而结构不同的化合物互为同分异构体(isomers)。A,B两种物质甲基在双键碳的不同位置,所以他们互为同分异构体(isomers)。
(iv) Draw the geometric isomer of compound B.
【答案如图】:
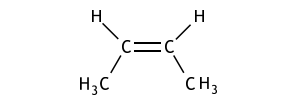
【分析】:由于双键的不可旋转当不同级别的官能团连在双键碳的同侧和异侧时会产生顺反异构
(geometric isomer)。
(v) Explain why compound B has a geometric isomer but compound A does not.
【答案】: There are two different groups bonded to each of the carbon atoms of the double bond in compound B
【分析】:只有双键碳两边连接的是不同的官能团时双键碳才有几何异构体(geometric isomer)。
(b) Compound C is an isomer of compounds A and B. Some reactions of compound C
are shown below.
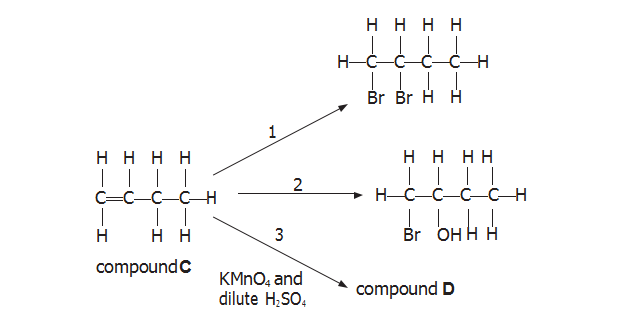
(i) Name the reagent(s) required for reaction 1.
【答案】:bromine
【分析】:烯烃的加成(addition ) 反应,双键断裂与卤素加成生成卤代烃(halohydrocarbon)。
(ii) Name the reagent(s) required for reaction 2.
【答案】: Bromine water
【分析】:烯烃须卤素溶液的加成,卤素和羟基(hydroxy)分别加到双键两侧。
(iii) Draw the displayed formula of compound D.
【答案如图】:
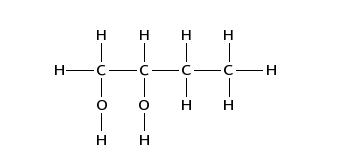
【分析】:烯烃在KMnO4 and dilute H2SO4条件下会发生加成反应生成邻二醇。
(C) Compound C also reacts with hydrogen chloride.
(i) Classify the type and mechanism of this reaction.
【答案】:Electropic Addition
【分析】:当烯烃相与HX和水反应生成邻二醇的时候,先加成生成一羟基卤代烃在取代生成邻二醇。
2 . The table below shows the experimental and calculated values for the lattice energy of sodium chloride and silver chloride.
| Compound | Lattice Energy / kJ mol−1 | |
| Experimental | Calculated | |
| sodium chloride | −780 | −770 |
| silver chloride | −905 | −833 |
(i) Write the equation for the lattice energy of sodium chloride. Include state symbols.
【答案】:Na+(g) + Cl (g) → NaCl(s)
【分析】:晶格能(lattice energy) 也可以说是破坏1mol晶体,使它变成完全分离的气态自由离子所需要消耗的能量。
(ii) Name the energy cycle used to calculate lattice energies from experimental data.
【答案】:Born-Haber (cycle)
【分析】:送分题,很明显答案是 Born-Haber (cycle)
(iii) Explain fully why the experimental and calculated values for the lattice energy of sodium chloride are similar, whereas those for silver chloride differ significantly.
【答案】:Sodium chloride is purely ionic, Silver chloride is partly covalent,
so small(er) electronegativity difference between Ag and Cl.
【分析】:因为钠和银之间的电负性相相氯和银之间的电负性(electronegativity)要大。
今天讲解的两道题分别考察了有机物和无机物中的相关知识点:
烃类的命名
有机化合物的分子式
同分异构体的概念
几何异构体的概念
烯烃的加成反应
晶格能的概念和影响因
伯恩哈勃循环

最新发布
© 2025. All Rights Reserved. 沪ICP备2023009024号-1









