- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
American Journal of Cardiovascular Drugs|中国高脂血症的药物治疗:单用辛伐他汀、...
Drug Treatment of Hyperlipidemia in Chinese Patients: Focus on the Use of Simvastatin and Ezetimibe Alone and in Combination

American Journal of Cardiovascular Drugs Review Article
Early Recent, Feb 04, 2019
10.1007/s40256-018-00317-1
本文由“天纳”临床学术信息人工智能系统自动翻译
Elevated serum low-density lipoprotein cholesterol (LDL-C) is a major risk factor for coronary heart disease (CHD). Many guidelines recommend LDL-C as a primary treatment target, and statins represent the cornerstone of treatment for lipid management. Recently revised guidelines recommend even more intense management of LDL-C, especially in patients at moderate and high risk. However, LDL-C levels in the Chinese population differ from those in Western populations, and the benefits and safety of the maximum allowable dose of statins have yet to be determined. Furthermore, in practice, many patients do not achieve the increasingly stringent LDL-C goals. Consequently, alternative approaches to lipid management are required. Combination therapy with ezetimibe and a statin, which have complementary mechanisms of action, is more effective than statin monotherapies, even at high doses. Several clinical studies have consistently shown that combination therapy with ezetimibe and simvastatin lowers LDL-C more potently than statin monotherapies. Moreover, the safety and tolerability profile of the combination therapy appears to be similar to that of low-dose statin monotherapies. This review discusses the role of simvastatin in combination with ezetimibe in controlling dyslipidemia in Chinese patients, particularly the efficacy and safety of combination therapy in light of recently published clinical data.
血清低密度脂蛋白胆固醇(LDL-C)升高是冠心病(CHD)的主要危险因素。许多指南建议将低密度脂蛋白胆固醇作为主要治疗目标,而他汀类药物是脂质管理治疗的基石。最近修订的指南建议对低密度脂蛋白胆固醇进行更为严格的管理,特别是对中高风险患者。然而,中国人群的低密度脂蛋白C水平与西方人群不同,他汀类药物最大允许剂量的益处和安全性尚未确定。此外,在实践中,许多患者没有达到日益严格的低密度脂蛋白胆固醇目标。因此,需要其他的脂质管理方法。依泽麦布联合他汀类药物具有互补的作用机制,即使在高剂量的情况下也比他汀类药物更有效。几项临床研究一致表明,联合使用依泽麦布和辛伐他汀比单独使用他汀类药物更能降低低密度脂蛋白胆固醇。此外,联合治疗的安全性和耐受性与低剂量他汀类单药治疗相似。本文根据近期发表的临床资料,探讨辛伐他汀联合依泽麦布治疗中国患者血脂异常的作用,特别是联合治疗的有效性和安全性。
Key Points
| Simvastatin and ezetimibe combination therapy was more effective than statin monotherapy among Chinese patients with dyslipidemia. |
| Data suggest that combination therapy (simvastatin and ezetimibe) is more effective than statin monotherapy at lowering elevated serum low-density lipoprotein cholesterol levels. |
| Combination therapy neither compromises the safety and efficacy of the drugs nor increases the risk of health complications in treated patients. |
1 Introduction to the Management of Lipid Disorders in Chinese Patients
Coronary heart disease (CHD) is the second leading cause of cardiovascular death in the Chinese population [1]. In 2013, the mortality rate due to cardiovascular diseases (CVDs) was 295.63 per 100,000 individuals in rural areas and 261.99 per 100,000 in urban areas. In total, 44.6% of deaths in rural areas and 42.51% of deaths in urban areas were caused by CVDs, rates that are higher than for any other disease, including cancer [2]. Accumulating evidence shows that the incidence of CHD is steadily increasing in China [3]. Measures for the prevention and treatment of CHD in China should be enacted without delay.
冠心病是中国人心血管死亡的第二大原因[1]。2013年,农村地区心血管疾病死亡率为每10万人295.63人,城市地区为每10万人261.99人。总的来说,农村地区44.6%的死亡和城市地区42.51%的死亡是由心血管疾病引起的,其发病率高于任何其他疾病,包括癌症[2]。积累的证据表明,我国冠心病发病率在稳步上升[3]。中国应及时制定预防和治疗冠心病的措施。
Elevated serum cholesterol levels will result in an increase in CVD events of about 9.2 million between 2010 and 2030 in China [4]. The prevalence of dyslipidemia in Chinese adults was as high as 34.0% overall and 35.1% and 26.3% in urban and rural areas, respectively [5]. Moreover, the role of low-density lipoprotein cholesterol (LDL-C) in the pathophysiology of CHD is acknowledged and well-understood, and the use of LDL-C-lowering medications has led to a significant reduction of cardiovascular risk in both primary and secondary prevention. Therefore, LDL-C is recommended as the primary treatment target for lipid management in many guidelines, such as the US National Cholesterol Education Program Adult Treatment Panel III (NCEP ATP III) guidelines [6], the 2012 Japan Atherosclerosis Society (JAS) guidelines [7], the 2013 American College of Cardiology (ACC)/American Heart Association (AHA) guidelines, the 2014/2015 National Lipid Association (NLA) recommendations for management of dyslipidemia in the context of evolving evidence [8], the 2016 European Society of Cardiology (ESC)/European Atherosclerosis Society (EAS) guidelines [9], and the 2016 Chinese guidelines for the management of adult dyslipidemia (revised edition) [10].
在中国,血清胆固醇水平升高将导致2010年至2030年间心血管疾病事件增加约920万[4]。中国成年人血脂异常的患病率总体高达34.0%,城乡地区分别高达35.1%和26.3%。此外,低密度脂蛋白胆固醇(LDL-C)在冠心病病理生理学中的作用已得到公认和充分理解,并且使用低密度脂蛋白C降低药物在一级和二级预防中已显著降低心血管风险。因此,在许多指南中,推荐低密度脂蛋白胆固醇(LDL-C)作为脂质管理的主要治疗目标,如美国国家胆固醇教育计划成人治疗小组(NCEP-ATP-III)指南[6]、2012年日本动脉粥样硬化学会(JAS)指南[7]、2013年美国心脏病学会(ACC)/美国心脏协会。(AHA)指南、2014/2015年国家脂质协会(NLA)在不断发展的证据背景下管理血脂异常的建议[8]、2016年欧洲心脏病学会(ESC)/欧洲动脉粥样硬化学会(EAS)指南[9]和2016年中国成人血脂异常管理指南(修订版)[10]
Since statins were introduced in the 1980s, they have emerged as one of the best-selling medication classes to date, with numerous trials demonstrating powerful efficacy in improving cardiovascular outcomes [11]. Statin therapy has become a cornerstone for the prevention and treatment of CHD and is generally safe and well-tolerated [9]. A number of large-scale clinical trials have demonstrated that statins substantially reduce cardiovascular morbidity and mortality as both primary and secondary prevention [12] and in high-risk patients [13]. The use of statins has revolutionized the management of CHD development risk, and more than 25 million individuals currently use statins worldwide [14]. However, despite the proven efficacy of statins in improving lipid profiles and reducing cardiovascular risk, a considerable proportion of statin-treated patients does not reach therapeutic LDL-C targets as defined by guidelines in China and other countries [15, 16, 17]. Many factors can influence the magnitude of response during lipid-altering therapy, including demographics (e.g., age, sex, race), metabolic disease (e.g., insulin resistance, diabetes mellitus, obesity, thyroid dysfunction), and treatment (e.g., statin type, dose, titration, combination therapy) [18].
自20世纪80年代开始使用他汀类药物以来,他汀类药物已成为迄今为止最畅销的药物类别之一,许多试验表明他汀类药物在改善心血管结局方面具有强大的疗效[11]。他汀类药物治疗已成为预防和治疗冠心病的基石,一般安全且耐受性良好[9]。许多大规模临床试验表明,他汀类药物作为一级和二级预防[12]和高危患者[13]都能显著降低心血管发病率和死亡率。他汀类药物的使用彻底改变了对冠心病发展风险的管理,目前全球有2500多万人使用他汀类药物[14]。然而,尽管他汀类药物在改善脂质分布和降低心血管风险方面的效果已被证实,但相当一部分他汀类药物治疗的患者没有达到中国和其他国家指导方针所定义的治疗性低密度脂蛋白胆固醇目标[15、16、17]。许多因素可影响脂质改变治疗期间的反应程度,包括人口统计学(例如,年龄、性别、种族)、代谢性疾病(例如,胰岛素抵抗、糖尿病、肥胖、甲状腺功能障碍)和治疗(例如,他汀类、剂量、滴定、联合治疗)[18]。
However, how to use statins reasonably and effectively in the Chinese population has always been controversial. The ESC/EAS guidelines recommend the use of high-intensity statins (equivalent to the maximum allowable dose) at the beginning of treatment, but the benefits and safety of the maximum allowable dose of statins have not yet been determined in the Chinese population [9, 19]. The genetics, lifestyle, and diet of Chinese individuals differ from those in European and US populations, and 80% of Chinese residents have LDL-C levels < 130 mg/dL, so fewer patients need high-dose statins to achieve a stronger lipid-lowering effect [10, 20]. The HPS2-THRIVE study demonstrated that Chinese individuals had a much higher risk of myopathy with simvastatin 40 mg monotherapy than did European subjects [21]. DYSIS (DYSlipidemia International Study), a large-scale cross-pal survey of dyslipidemia management in patients receiving lipid-lowering agents in China, showed that the LDL-C control rates in high-risk and very high-risk populations were 54.8% and 39.7%, respectively. It also showed that increasing the statin dose did not increase the medication compliance rate [22]. Therefore, most Chinese patients may not respond to the intensive statin dosage suggested by European guidelines. This highlights the need to explore an appropriate cholesterol-lowering therapy for high-risk patients in China. The CHILLAS trial did not show that high-intensity statins could provide more benefit to patients with acute coronary syndrome (ACS) in China [23], and the safety of high-intensity statins is a concern in the Chinese population. An increasing number of studies show that high-intensity statin treatment is accompanied by a higher risk of myopathy and elevated liver enzymes, which is also more prominent in the Chinese population. The study found no linear relationship between the efficacy and dosage of statins. The lipid-lowering efficacy of statins is characterized by initial lipid-lowering efficacy at the start of treatment. However, doubling the dose only decreased LDL-C by a further approximately 6%, and the drug cost increased proportionally. Therefore, it is recommended [10] that moderate-strength statins be initially used, with the dosage appropriately adjusted according to the lipid-lowering effect and tolerance. If the cholesterol level is not to standard, a safe and effective lipid-lowering effect can be obtained by combining with other lipid-lowering drugs (class I recommendation, class B evidence) [10].
然而,如何在中国人群中合理有效地使用他汀类药物一直存在争议。ESC/EAS指南建议在治疗开始时使用高强度他汀类药物(相当于最大允许剂量),但中国人群尚未确定他汀类药物最大允许剂量的益处和安全性[9,19]。中国人的遗传、生活方式和饮食与欧美人群不同,80%的中国居民的低密度脂蛋白C水平为<130 mg/dl,因此较少的患者需要高剂量他汀类药物来达到更强的降脂效果[10,20]。hps2-threeve研究表明,使用辛伐他汀40 mg单药治疗的中国人比欧洲受试者有更高的肌病风险[21]。Dysis(Dyslipidemia International Research)是一项对中国接受降脂药物治疗的患者进行的大规模横断面调查,结果表明,高危人群和高危人群的低密度脂蛋白胆固醇控制率分别为54.8%和39.7%。研究还表明,增加他汀类药物的剂量并不会增加药物依从率[22]。因此,大多数中国患者可能对欧洲指南建议的强化他汀类药物剂量没有反应。这突显出在中国,有必要为高危患者探索一种适当的降胆固醇疗法。Chillas试验并未显示高强度他汀类药物对中国急性冠脉综合征(ACS)患者有更大的益处[23],高强度他汀类药物的安全性是中国人群的关注点。越来越多的研究表明,高强度他汀类药物治疗伴随着更高的肌病风险和肝酶升高,这在中国人群中也更为突出。研究发现他汀类药物的疗效与剂量之间没有线性关系。他汀类药物的降脂效果以治疗开始时的初始降脂效果为特征。然而,双倍剂量只会使低密度脂蛋白胆固醇进一步降低约6%,药物成本也相应增加。因此,建议[10]首先使用中等强度的他汀类药物,并根据降脂效果和耐受性适当调整剂量。如果胆固醇水平不符合标准,可与其他降脂药物联合使用(I级推荐,B级证据)获得安全有效的降脂效果[10]。
For high-risk patients who do not achieve adequate LDL-C lowering, use of a non-statin drug in combination with statins is recommended as an alternative therapeutic option. Ezetimibe was the first lipid-lowering agent found to inhibit the intestinal uptake of dietary and biliary cholesterol without affecting the absorption of fat-soluble nutrients [24]. When combined with statin therapy, the resulting complementary inhibition of cholesterol absorption and synthesis is more effective than statin monotherapy at reducing LDL-C, even at high statin doses. Furthermore, by combining ezetimibe with a statin, the requirement for statin dose titration can be reduced, thereby minimizing the risk of dose-related adverse events (e.g., myopathy, rhabdomyolysis, hepatotoxicity) [25]. Simvastatin with ezetimibe is available as a fixed-dose treatment that leads to a significant reduction in serum concentrations of the atherogenic LDL-C [26]. This review discusses the role of simvastatin in combination with ezetimibe in controlling dyslipidemia in Chinese patients in light of recently published clinical data.
对于未达到适当降低低密度脂蛋白胆固醇水平的高危患者,建议将非他汀类药物与他汀类药物联合使用作为替代治疗方案。依泽麦布是第一种降脂剂,被发现在不影响脂溶性营养素吸收的情况下抑制肠道对膳食和胆汁胆固醇的吸收[24]。当与他汀类药物联合治疗时,所产生的对胆固醇吸收和合成的补充抑制比他汀类药物单独治疗在降低低密度脂蛋白胆固醇(LDL-C)方面更有效,即使是在高他汀类药物剂量下。此外,通过将依泽麦布与他汀类药物结合,可以降低他汀类药物剂量滴定的要求,从而将剂量相关不良事件(例如肌病、横纹肌溶解症、肝毒性)的风险降至最低[25]。辛伐他汀联合依泽替米贝可作为一种固定剂量的治疗,导致动脉粥样硬化性低密度脂蛋白胆固醇的血清浓度显著降低[26]。本文根据近期发表的临床资料,探讨辛伐他汀联合依泽麦布对中国患者血脂异常的控制作用。
2 Treatment Aims and Low-Density Lipoprotein Cholesterol (LDL-C) Targets
LDL-C target levels are defined according to the patient’s cardiovascular risk. In most people, atherosclerotic CVD (ASCVD) is the result of a number of risk factors, so all current guidelines on the prevention of CVD in clinical practice recommend the assessment of clinically manifest ASCVD and cardiovascular risk [9].
根据患者的心血管风险确定低密度脂蛋白胆固醇的目标水平。在大多数人中,动脉粥样硬化性脑血管病(ascvd)是多种危险因素的结果,因此,所有当前临床实践中有关预防脑血管病的指南都建议对临床表现为ascvd和心血管风险进行评估[9]。
Several large clinical trials have shown that every 1 mmol/L reduction in LDL-C is associated with a 23% decrease in CVD risk [27]. According to the European guidelines, the presence of ASCVD, such as a history of acute myocardial infarction or stroke (i.e., secondary prevention), defines an LDL-C target of < 1.8 mmol/L (70 mg/dL). In primary prevention for patients with type 2 diabetes mellitus (T2DM), or multiple risk factors leading to the estimation of high cardiovascular risk, an LDL-C level of < 2.6 mmol/L (100 mg/dL) should be targeted. If these absolute treatment goals are not reached, LDL-C levels should be at least halved [28].
几项大型临床试验表明,低密度脂蛋白胆固醇每降低1毫摩尔/升与心血管疾病风险降低23%有关[27]。根据欧洲指南,存在血管紧张性血管疾病,如急性心肌梗死或中风史(即二级预防),定义了<1.8 mmol/L(70 mg/dl)的低密度脂蛋白胆固醇目标。在2型糖尿病(t2dm)患者的一级预防中,或导致高心血管风险评估的多个风险因素中,应针对低密度脂蛋白胆固醇水平<2.6毫摩尔/升(100毫克/分升)。如果没有达到这些绝对治疗目标,低密度脂蛋白胆固醇水平应至少减半[28]。
The 2013 AHA/ACC guidelines do not mention any absolute target LDL-C levels, instead recommending high-intensity statin treatment to reduce LDL-C levels by ≥ 50% in patients with manifest ASCVD or high estimated risk and by 30–50% in patients with moderate risk. The new pooled cohort equations risk calculator qualifies a larger population for treatment with statins, which has been controversial [29, 30].
2013年美国心脏协会(AHA/ACC)指南并未提及任何绝对目标低密度脂蛋白胆固醇水平,而是建议高强度他汀类药物治疗,以降低低密度脂蛋白胆固醇水平≥50%的患者有明显的心血管疾病或高估计风险,30-50%的患者有中度风险。新的集合队列方程风险计算器使更大的人群有资格接受他汀类药物治疗,这一直是有争议的[29,30]。
The 2016 revised Chinese guidelines suggest that both non-high-density lipoprotein cholesterol (HDL-C) and LDL-C be considered targets for lipid-altering therapy, and define therapeutic goals (Table 1) [10].
2016年修订的中国指南表明,非高密度脂蛋白胆固醇(HDL-C)和低密度脂蛋白胆固醇(LDL-C)均被视为脂质改变治疗的目标,并确定治疗目标(表1)[10]。
Table 1
| Risk category | LDL-C | Non-HDL-C |
| Low or moderate | < 3.4 mmol/L (130 mg/dl) | < 4.1 mmol/L (160 mg/dl) |
| High | < 2.6 mmol/L (100 mg/dl) | < 3.4 mmol/L (130 mg/dl) |
| Very high | < 1.8 mmol/L (70 mg/dl) | < 2.6 mmol/L (100 mg/dl) |
HDL-C high-density lipoprotein cholesterol, LDL-C low-density lipoprotein cholesterol
Studies in other countries have shown that the poor LDL-C control rate for high-risk patients treated in general practice is a global problem [31, 32, 33]. In South Africa, the control rate for very high-risk patients was reported to be only 26.5%. Similarly, 67.2% of very high-risk patients in Lebanon and Jordan missed the goal [34, 35]. Only 68% of patients in a high-risk cohort in Japan reached guideline-recommended LDL-C goals, and only 2% were treated with a statin, suggesting that statins are underutilized in high-risk Japanese populations [36]. Even in some developed regions, such as Europe and Canada, 45.3% of patients with diabetes were not at their LDL-C goal [37]. Despite the importance of achieving LDL-C targets, little improvement in LDL-C control has been realized for high-risk patients in China. DYSIS found that 62.5% of high-risk and very high-risk patients missed the LDL-C therapeutic goal in 2013 [22], which is only slightly better than the 74% reported for 2002 [38].
其他国家的研究表明,一般情况下接受治疗的高危患者低密度脂蛋白胆固醇控制率低是一个全球性问题[31,32,33]。在南非,据报告,极高危患者的控制率仅为26.5%。同样,67.2%的黎巴嫩和约旦的高危患者没有达到目标[34,35]。在日本高风险队列中,只有68%的患者达到了指导性建议的低密度脂蛋白胆固醇目标,只有2%的患者接受了他汀类药物治疗,这表明他汀类药物在高风险日本人群中的应用不足[36]。即使在一些发达地区,如欧洲和加拿大,45.3%的糖尿病患者没有达到他们的低密度脂蛋白胆固醇目标[37]。尽管实现低密度脂蛋白胆固醇(LDL-C)目标很重要,但我国高风险患者的低密度脂蛋白胆固醇(LDL-C)控制并未得到改善。Dysis发现,62.5%的高风险和非常高风险患者在2013年没有达到低密度脂蛋白胆固醇治疗目标[22],仅略好于2002年的74%(38)。
Failure to attain therapeutic LDL-C goals may be attributed to several factors in China [16]. First, patient and physician adherence to lipid management remains poor, not least because hypercholesterolemia is an asymptomatic chronic condition, but also because some patients experience minor side effects with statin use. In addition, prescription rates for statins are < 50%, even for high- or very high-risk patients [39]. Second, sex and obesity are strong risk factors for not reaching therapy goals, because women have a higher prevalence of hypercholesterolemia, and obese patients are often resistant to therapy and may require both combination drug therapy and lifestyle interventions. Finally, combination therapy as an alternative to high-dose statin monotherapy is often underused in clinical practice, primarily because of concerns about drug interactions and major adverse events. Cost is only a minor consideration in China, because most statins are covered by national medical insurance, and patients can receive full reimbursement for simvastatin. A survey of primary care physicians in 2010 [40] found that only 24% and 14% of participants could report the optimal LDL-C levels for patients with CHD and diabetes, respectively. Therefore, educational interventions to improve physicians’ knowledge of lipid guidelines should be emphasized [17]. Creation of a national program to raise public awareness is also imperative.
未能达到治疗性低密度脂蛋白胆固醇(LDL-C)的目标可能归因于中国的几个因素[16]。首先,患者和医生对脂质管理的依从性仍然很差,这不仅是因为高胆固醇血症是一种无症状的慢性疾病,而且是因为一些患者在使用他汀类药物时出现轻微副作用。此外,他汀类药物的处方率为<50%,即使是高风险或非常高风险的患者也是如此[39]。其次,性别和肥胖是达不到治疗目标的强烈危险因素,因为女性高胆固醇血症的患病率较高,肥胖患者往往对治疗有抵抗力,可能需要联合药物治疗和生活方式干预。最后,组合疗法作为高剂量他汀类药物单一疗法的替代方案,在临床实践中经常被低估,主要是因为担心药物相互作用和主要不良事件。在中国,成本只是一个很小的考虑因素,因为大多数他汀类药物都由国家医疗保险覆盖,患者可以得到辛伐他汀的全额补偿。2010年对初级保健医师进行的一项调查[40]发现,只有24%和14%的参与者可以分别报告冠心病和糖尿病患者的最佳低密度脂蛋白C水平。因此,应强调教育干预以提高医师对脂质指南的认识[17]。建立一个提高公众意识的国家计划也是必要的。
3 Cholesterol Metabolism and Pharmacology of Statins and Ezetimibe
Cholesterol metabolism consists of a complex set of interactive input and output fluxes providing the large membrane-bound cholesterol pool with sufficient cholesterol molecules. Serum cholesterol is derived from the biosynthesis (endogenous pathway) and intestinal uptake (exogenous pathway) of dietary and biliary cholesterol [41]. Cholesterol is also catabolized to produce steroid hormones and bile acids [42]. Synthesis of cholesterol occurs in the cytoplasm and membrane of the endoplasmic reticulum of virtually all tissues in humans, including the liver, intestine, adrenal cortex, and reproductive tissues. Most of the circulating cholesterol is derived from internal manufacture rather than diet. The liver produces approximately 70% of total cholesterol (TC) in the body and when the liver can no longer produce it, blood cholesterol levels will subsequently fall [43]. The chemical pathway producing cholesterol in all cells begins with condensation of acetyl-CoA with acetoacetyl-CoA to form 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) in a reaction catalyzed by HMG-CoA synthase (Fig. 1). The HMG-CoA reductase (HMGCR) enzyme then converts HMG-CoA to mevalonate, which is the first rate-limiting step in this pathway [44]. This rate-limiting step is the conversion of HMG-CoA to CoA and mevalonate by a four-electron reductive deacylation process. After mevalonate is generated, multiple reactions follow to finally produce cholesterol. Statins are competitive, reversible inhibitors of HMGCR, the rate-limiting enzyme of cholesterol biosynthesis [45]. Statins, which modulate only endogenous cholesterol, inhibit biosynthesis of cholesterol, deplete intracellular pools, and enhance removal of plasma LDL-C [46], leading to significant reductions in serum LDL-C [47].
胆固醇代谢由一组复杂的相互作用的输入和输出通量组成,为大的膜结合胆固醇池提供足够的胆固醇分子。血清胆固醇来源于饮食和胆汁胆固醇的生物合成(内源性途径)和肠道摄取(外源性途径)。胆固醇也被分解代谢产生类固醇激素和胆汁酸[42]。胆固醇的合成发生在人体几乎所有组织(包括肝、肠、肾上腺皮质和生殖组织)内质网的细胞质和膜中。循环中的大部分胆固醇来自于内部制造,而不是饮食。肝脏在体内产生大约70%的总胆固醇(TC),当肝脏不能再产生时,血液胆固醇水平随后会下降[43]。在所有细胞中产生胆固醇的化学途径开始于乙酰辅酶A与乙酰乙酰辅酶A的缩合,在HMG辅酶A合成酶催化的反应中形成3-羟基-3-甲基戊二酰辅酶A(HMG辅酶A)(图1)。然后,HMG-CoA还原酶(HMGCR)酶将HMG-CoA转化为甲羟戊酸盐,这是该途径的第一个限速步骤[44]。这个限速步骤是通过四电子还原脱酰过程将HMG-CoA转化为CoA和甲戊酸盐。生成甲戊酸酯后,会发生多种反应,最终产生胆固醇。他汀类药物是胆固醇生物合成的限速酶HMGCR的竞争性可逆抑制剂[45]。他汀类药物只调节内源性胆固醇,抑制胆固醇的生物合成,耗尽细胞内池,增强血浆低密度脂蛋白胆固醇的去除[46],导致血清低密度脂蛋白胆固醇的显著降低[47]。
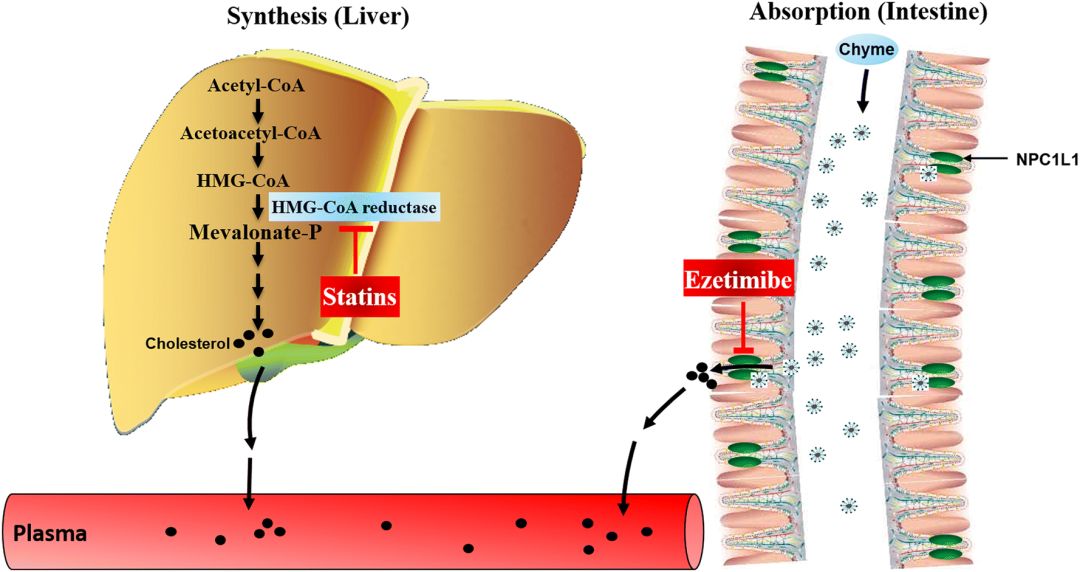
Fig. 1Effects of statins and ezetimibe on cholesterol. HMG-CoA 3-hydroxy-3-methylglutaryl coenzyme A, NPC1L1 Niemann-Pick C1-like 1
Statins exert lipid-lowering effects in two ways. The open ring portion of the chemical structure of inhibitors orally administered as active hydroxy acid forms (atorvastatin, fluvastatin, pitavastatin, pravastatin, and rosuvastatin) or prodrugs with a lactone ring (lovastatin and simvastatin) is similar to HMG-CoA [45]. The compounds therefore inhibit formation of mevalonate by competing with HMG-CoA, interrupting endogenous cholesterol synthesis and leading to a reduction in intracellular cholesterol synthesis. Simvastatin is administered as a lactone prodrug and is subsequently transformed to active metabolites. In vivo, lactone prodrugs are enzymatically hydrolyzed to their hydroxy acid pharmacophores in the liver to achieve pharmacological activity [48]. Alternately, reduced endogenous cholesterol synthesis in serum leads to increased LDL-C receptor expression on the surface of hepatocytes. Increased LDL-C receptor expression causes increased uptake of plasma LDL-C and consequently decreases plasma LDL-C levels [49].
他汀类药物有两种降脂作用。作为活性羟基酸形式(阿托伐他汀、氟伐他汀、匹他伐他汀、普伐他汀和瑞舒伐他汀)或具有内酯环(洛伐他汀和辛伐他汀)的前药,口服抑制剂的化学结构的开环部分与HMG-CoA相似[45]。因此,这些化合物通过与HMG-CoA竞争,中断内源性胆固醇合成并导致细胞内胆固醇合成减少,从而抑制甲戊酸盐的形成。辛伐他汀作为内酯前药给药,随后转化为活性代谢物。在体内,内酯前药被酶水解为其在肝脏中的羟基酸药物载体,以达到药理活性[48]。另外,血清中内源性胆固醇合成减少导致肝细胞表面LDL-C受体表达增加。LDL-C受体表达增加导致血浆LDL-C摄取增加,从而降低血浆LDL-C水平[49]。
The Niemann-Pick C1-like 1 (NPC1L1) sterol transporter, located at the intestinal wall brush-border membrane and canalicular membrane of the hepatocyte, plays a vital role in cholesterol absorption in the intestine and liver; hepatic NPC1L1 promotes the reabsorption of cholesterol from bile and decreases the loss of cholesterol from the liver [50]. Ezetimibe, a selective cholesterol absorption inhibitor, can effectively inhibit intestinal absorption of cholesterol from diet, along with bile and hepatic absorption, by binding to the NPC1L1 receptor, resulting in low cholesterol levels in vivo (Fig. 1) [51]. Ezetimibe monotherapy can reduce LDL-C levels by approximately 18%, as well as triglyceride and apolipoprotein B (ApoB) levels by approximately 5% and 15%, respectively. Significant elevation of HDL-C has also been observed [52, 53].
Niemann-Pick C1-like 1(NPC1L1)固醇转运蛋白位于肝细胞的肠壁刷状缘膜和小管膜上,在肠和肝内胆固醇吸收中起着至关重要的作用;肝NPC1L1促进胆汁中胆固醇的再吸收,减少肝内胆固醇的流失[50]。依泽麦布是一种选择性的胆固醇吸收抑制剂,通过与NPC1L1受体结合,可以有效地抑制饮食中胆固醇的肠道吸收,以及胆汁和肝脏的吸收,从而降低体内胆固醇水平(图1)[51]。依泽麦布单一疗法可使低密度脂蛋白C水平降低约18%,甘油三酯和载脂蛋白B(apob)水平分别降低约5%和15%。还观察到HDL-C显著升高[52,53]。
The mechanisms by which NPC1L1 mediates cholesterol uptake and ezetimibe inhibits this process are only partially understood. NPC1L1 mediates cholesterol uptake via vesicular endocytosis, as cholesterol specifically promotes the incorporation of NPC1L1 into clathrin-coated vesicles [54]. Microfilaments and a complex of clathrin and its adapter protein AP2 mediate the endocytosis. At the same time, ezetimibe prevents NPC1L1 from incorporating into clathrin-coated vesicles and thus inhibits cholesterol uptake [54]. Recently, a mechanism for efficient free cholesterol absorption, the formation of cholesterol-enriched membrane microdomains positive for flotillins (NPC1L1 lipid raft proteins), was elucidated [55]. Ezetimibe may reduce plasma cholesterol by inhibiting NPC1L1 function in both intestine and liver, and hepatic NPC1L1 may protect the body from excessive biliary loss of cholesterol [56].
NPC1L1介导胆固醇摄取和依泽替米贝抑制这一过程的机制只有部分了解。NPC1L1通过囊泡内吞作用介导胆固醇摄取,因为胆固醇特别促进NPC1L1并入网格蛋白包膜囊泡[54]。微丝体和网格蛋白及其结合蛋白ap2的复合体介导内吞作用。同时,依泽替米贝阻止NPC1L1与网格蛋白包膜囊泡结合,从而抑制胆固醇摄取[54]。最近,一个有效的自由胆固醇吸收机制,即形成富含胆固醇的膜微结构域,对flotillins(NPC1L1脂质筏蛋白)呈阳性,被阐明[55]。依泽替米贝可通过抑制肠和肝中的NPC1L1功能来降低血浆胆固醇,肝中的NPC1L1可保护机体免受过度胆道胆固醇损失[56]。
By inhibiting two independent pathways of plasma cholesterol generation—the exogenous pathway of cholesterol absorption from the gut (via ezetimibe) and the endogenous pathway of cholesterol biosynthesis in the liver (via simvastatin)—combination therapy may offer an efficacious strategy for the prevention and treatment of lipid disorders (Fig. 1). A number of clinical studies have consistently demonstrated that the simvastatin/ezetimibe combination provides more potent LDL-C lowering than statin monotherapy [18]. Thus, simvastatin/ezetimibe combination therapy reduces the need for statin dose titration and potentially decreases the adverse events associated with high-dose statin monotherapy. The SEAS (Simvastatin and Ezetimibe in Aortic Stenosis) study was a randomized, double-blind, placebo-controlled, multicenter study of a minimum 4 years’ duration investigating the effect of lipid lowering with an ezetimibe/simvastatin combination (10 mg/40 mg per day) in Western patients with asymptomatic aortic stenosis (AS). The study found that combination therapy with ezetimibe and a statin lowered LDL-C more than statins alone [57].
通过抑制血浆胆固醇生成的两个独立途径,从肠道吸收胆固醇的外源途径(通过ezetimibe)和肝内胆固醇生物合成的内源性途径(通过辛伐他汀)-联合治疗可为预防和治疗血脂紊乱提供有效的策略(图(1)。许多临床研究一直证明辛伐他汀/依泽替米贝联合用药比他汀单药疗法能更有效地降低低密度脂蛋白胆固醇[18]。因此,辛伐他汀/依泽替米贝联合治疗减少了他汀剂量滴定的需要,并可能减少与高剂量他汀单药治疗相关的不良事件。SEAS(主动脉瓣狭窄中的辛伐他汀和依泽替米贝)研究是一项随机、双盲、安慰剂对照、多中心研究,至少持续4年,研究依泽替米贝/辛伐他汀联合用药(10 mg/40 mg/天)对西方无症状主动脉瓣狭窄(AS)患者降脂的影响。研究发现,与单独使用依泽替米贝和他汀类药物相比,联合使用依泽替米贝和他汀类药物可降低低密度脂蛋白胆固醇[57]。
4 Pharmacokinetics of Simvastatin Alone or in Combination with Ezetimibe in China
Simvastatin is known to be absorbed in the intestine after administration as an inactive prodrug, and its absorption range is 80–85%. Food intake does not affect its absorption. In a study of healthy Chinese subjects, the time to peak plasma concentration (Tmax) was 0.7–3.0 h [58, 59]. In plasma, more than 95% of simvastatin is bound to plasma protein. Simvastatin undergoes phase I and phase II drug metabolism after oral administration. It can be readily converted into its active metabolite, simvastatin hydroxy acid, which serves as a potent competitive inhibitor of HMGCR by carboxylesterase or, without the enzyme, in the intestinal mucosa, plasma, and liver [60]. Simvastatin is then principally metabolized by phase I drug-metabolizing enzymes, cytochrome P450 (CYP)-3A4 and CYP3A5, in hepatic microsomes, where the former plays a major role [61]. Although simvastatin hydroxy acid can be metabolized by CYP3A4, CYP3A5, CYP2C8, and uridine 5ʹ-diphospho-glucuronosyltransferase (UGT), CYP3A4 is the most important participant [62, 63]. Moreover, simvastatin has a higher liver selectivity than simvastatin hydroxy acid [64], promoting its metabolism. As simvastatin undergoes extensive first-pass metabolism in the liver, its bioavailability is not believed to exceed 5% [65]. Simvastatin is glucuronidated via UGT 1A1 and 1A3 [61] and then excreted mainly via feces (60%) and urine (13%) [65]. Its elimination half-life (t½) in healthy Chinese subjects was reported to be 2.4–6.1 h [58].
众所周知,辛伐他汀作为一种不活跃的前药在肠内被吸收,其吸收范围为80-85%。食物摄入不会影响其吸收。在一项对中国健康受试者的研究中,血浆浓度峰值时间(tmax)为0.7-3.0_h[58,59]。在血浆中,95%以上的辛伐他汀与血浆蛋白结合。辛伐他汀口服后进入一期和二期药物代谢。它可以很容易地转化为其活性代谢物辛伐他汀羟基酸,通过羧酸酯酶或在没有酶的情况下,在肠粘膜、血浆和肝脏中作为HMGCR的有效竞争抑制剂[60]。辛伐他汀主要由肝微粒体中的第一阶段药物代谢酶细胞色素P450(cyp)-3A4和cyp3A5代谢,前者起主要作用[61]。尽管辛伐他汀羟基酸可由cyp3a4、cyp3a5、cyp2c8和尿苷5-二磷酸葡萄糖醛酸转移酶(ugt)代谢,但cyp3a4是最重要的参与者[62,63]。此外,辛伐他汀比辛伐他汀羟基酸具有更高的肝脏选择性[64],促进其代谢。由于辛伐他汀在肝脏中经历了广泛的一次代谢,其生物利用度不超过5%[65]。辛伐他汀通过ugt 1a1和1a3进行葡萄糖醛酸化[61],然后主要通过粪便(60%)和尿液(13%)排出[65]。据报道,中国健康受试者的消除半衰期(t½)为2.4-6.1_h[58]。
Following oral administration, ezetimibe can also be rapidly absorbed in the liver and intestine and simultaneously extensively metabolized to its active ezetimibe-glucuronide form by glucuronidation of UGT [66]. Tmax of total ezetimibe (free ezetimibe and ezetimibe-glucuronide) is 1–2 h post-administration [67]. Plasma protein binding rates of ezetimibe and ezetimibe-glucuronide conjugates are usually considered to be around 90% [68]. Ezetimibe and ezetimibe-glucuronide are excreted predominantly in the bile, while a small portion is excreted by the kidney. Because of enterohepatic recycling, the t½ of ezetimibe and ezetimibe-glucuronide is approximately 22 h [68]. As ezetimibe is metabolized primarily by glucuronidation rather than oxidation of CYP, there are no significant interactions with other medications metabolized by the CYP pathway, such as simvastatin [69]. Thus, studies of the ezetimibe/simvastatin combination have become a focus of physicians and researchers.
口服后,依泽替米贝也能迅速被肝脏和肠道吸收,并通过ugt的胶凝作用同时广泛代谢为其活性的依泽替米贝胶凝物形式[66]。总依泽替米贝(游离依泽替米贝和依泽替米贝葡萄糖醛酸苷)的Tmax为给药后1–2小时[67]。依泽替米贝和依泽替米贝-葡萄糖醛酸结合物的血浆蛋白结合率通常被认为是90%左右[68]。依泽替米贝和依泽替米贝葡萄糖醛酸苷主要在胆汁中排出,而一小部分由肾脏排出。由于肠肝循环,ezetimibe和ezetimibe glucuronide的t½约为22_h[68]。由于依泽替米贝主要通过葡萄糖醛酸化而不是CYP氧化进行代谢,因此与其他由CYP途径代谢的药物(如辛伐他汀)没有明显的相互作用[69]。因此,对依泽替米贝/辛伐他汀联合用药的研究已成为医生和研究人员的焦点。
A recent study of the ezetimibe/simvastatin 10 mg/40 mg combination in healthy Chinese subjects found that the pharmacokinetic parameters for ezetimibe and simvastatin were assessed by determining total ezetimibe, free ezetimibe, simvastatin, and simvastatin acid concentrations using a validated liquid chromatography-tandem mass spectrometry method. The results showed the following pharmacokinetic parameters of total ezetimibe and free ezetimibe: peak plasma concentration (Cmax) 81.56 ± 26.62 and 9.40 ± 6.17 ng/mL, Tmax 0.93 ± 0.30 and 1.25 ± 1.27 h, and t½ 24.32 ± 13.27 and 18.90 ± 9.66 h, respectively. The pharmacokinetic parameters for simvastatin and simvastatin hydroxy acid following a single dose were Cmax 11.92 ± 5.50 and 3.37 ± 1.78 ng/mL, Tmax 0.98 ± 0.28 and 3.73 ± 1.68 h, and t½ 4.19 ± 1.81 and 7.65 ± 7.96 h, respectively [70]. The pharmacokinetic characteristics of ezetimibe in healthy Chinese subjects were generally consistent with those in non-Chinese subjects [68]. However, in our study, the area under the concentration–time curve (AUC) and Cmax were higher and t½ was longer than results reported in 24 healthy Caucasian subjects [71]. These differences may be attributed to physical characteristics, and genetic polymorphisms may also be involved.
最近对中国健康受试者联合使用依泽替米贝/辛伐他汀10 mg/40 mg的研究发现,依泽替米贝和辛伐他汀的药代动力学参数是通过使用经验证的液相色谱串联质谱法测定依泽替米贝、游离依泽替米贝、辛伐他汀和辛伐他汀酸的浓度来评估的。霍德。结果表明:总依泽替米贝和自由依泽替米贝的药代动力学参数为:血浆峰值浓度(Cmax)81.56峰值浓度(Cmax)26.62和9.40 6.17 ng/ml、Tmax 0.93 0.30和1.25和自由依泽替米贝的药代动力学参数为:血浆峰值浓度(Cmax)81.56 8201; 26.62和9.40 6.17分别。单次给药后辛伐他汀和辛伐他汀羟基酸的药代动力学参数分别为Cmax 11.92 1.78 ng/ml、Tmax 0.98.28和3.73 1.68 h和T½4.19.81和7.65 7.96 h,分别为(分别为[max 11.92页]。依泽替米贝在健康中国受试者体内的药动学特征与非中国受试者的药动学特征基本一致[68]。然而,在我们的研究中,浓度-时间曲线(AUC)和Cmax下的面积较高,t½比24名健康白种人报告的结果长[71]。这些差异可能归因于物理特性,也可能涉及遗传多态性。


最新发布
© 2025. All Rights Reserved. 沪ICP备2023009024号-1








