- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
「每日一练」ALevel化学例题解析及练习
每日一练ALevel Chemistry
今日知识点:Ionisation energy and valence electrons
例题
The table gives the successive ionisation energies for an element X.
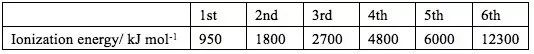
What could be the formula of the chloride of X
A XCl
B XCl2
C XCl3
D XCl4
解析
Answer: C
Definition of Ionisation Energy
A → A+ + e-
The energy required for the above reaction, or removing an electron. (挪走一个电子需要的能量)
Based on the table, there is a “jump” from the 5th to the 6th ionization energies, so there are 5 valence electrons.
5th 和6th 之间的数字变化比较大, 说明挪走第6个电子的需要很高能量,很困难,即挪走5个电子后达到8电子稳定结构,所以最外层有5个电子。
If X has 5 valence electrons, it needs to get an additional 3 electrons to form an Octect. Since each chlorine can provide 1 electron to form a bonding pair, 3 Cl is needed, forming XCl3.
氯化物中心的X需要形成8电子稳定结构,所以还需要额外和Cl共用3个电子,每个Cl只能共用1个电子,所以需要3个Cl.
下面我们为大家准备了一道同类型的题目,请大家一起来试试解答。
Your turn
Three successive elements in the Periodic Table have first ionization energies which have the pattern shown in the diagram.
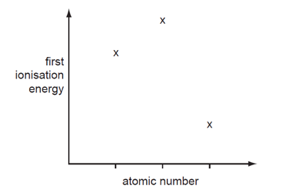
What could be the first element of the sequence?
A C
B N
C F
D Na
.
.
.
.
.
.
.
.
.
.
.
.
.
Correct Answer:C


最新发布
© 2025. All Rights Reserved. 沪ICP备2023009024号-1








