- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
2013 AP Statistics统计学真题Practice Exam系列之简答题免费下载
历年AP Statistics统计学系列
真题与答案下载

翰林国际教育全网首发
力争超快速发布最全资料
助你在升学路上一帆风顺
为你的未来保驾护航
2013 AP Statistics Practice Exam Free-Response Questions Free Download
2013 AP统计学模考简答题部分免费下载
此套题仅Section II含有简答题
共计1小时30分钟,共6题
占总分50%
每道大题可能含有不同数量的小题
其中Part A建议用时1小时05分钟,共5题,占Section II总分75%
Part B建议用时25分钟,共1题,占Section II总分25%
可以使用图形计算器
考试时会提供常用公式表
完整版下载链接见文末
部分真题预览:
Part A: 1)A hospital administrator noticed that the first nonemergency surgery scheduled each day often started late. If the first scheduled surgery got delayed, then all of the other surgeries scheduled for that day also got delayed. For three weeks (a total of 15 days) the administrator recorded how many minutes past the scheduled time the first surgery began each weekday. The data are shown in the table below. The administrator sent a memo to the hospital’s entire surgical staff to ask that everyone work to reduce the delay in the starting time for the first nonemergency surgery each day.
The administrator sent a memo to the hospital’s entire surgical staff to ask that everyone work to reduce the delay in the starting time for the first nonemergency surgery each day.
The administrator recorded how many minutes past the scheduled starting time the first scheduled surgery began each weekday for the three weeks after the memo was sent out. The data are shown in the table below. A negative number in the table indicates that the surgery started earlier than the scheduled time.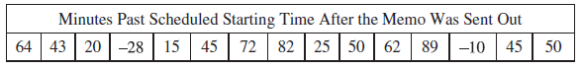 The dotplots below display the distributions of minutes past the scheduled starting time before the memo went out and after the memo went out.
The dotplots below display the distributions of minutes past the scheduled starting time before the memo went out and after the memo went out.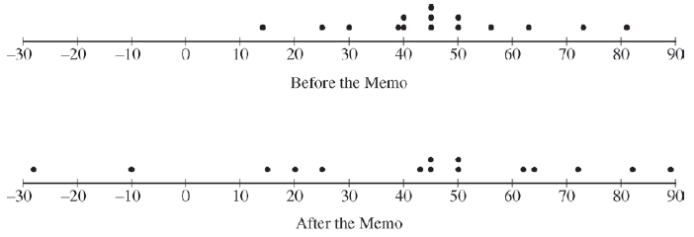
- Based only on the dotplots, does it appear that the distribution of minutes past the scheduled starting time changed after the memo was sent? Explain.
- The hospital administrator wants to perform a two-sample t-test to determine whether the average number of minutes past the scheduled starting time changed after the memo was sent. State the conditions for that test. For each condition, comment on whether it appears to be met.
Part B: 6)A drug company currently sells a prescription pain reliever that has been shown to be effective at lowering arthritis pain. However, since the drug also causes stomach irritation in some patients, the company has created a new formulation that it hopes will reduce that side effect.
To see if the new formulation reduces the occurrence of stomach irritation for users of the pain reliever, the company conducted a small preliminary study to compare the new formulation with the current pain reliever. In the preliminary study of 100 subjects with arthritis, 50 were randomly assigned to take the current pain reliever and 50 were randomly assigned to take the new formulation.
Patient responses at the end of the study are summarized in the table below.
- Do the data from the preliminary study indicate, at the 10 percent level of significance, that the new formulation helps to reduce the proportion of patients with stomach irritation compared to the current pain reliever? (The conditions for inference have been checked and verified.)
If you need more room for your work for part (a), use the space below. - Based on your conclusion in part (a), which type of error, Type I or Type II, is possible? Describe the consequences of each error in the context of this study.

最新发布
© 2026. All Rights Reserved. 沪ICP备2023009024号-1









